Infection
Risk of hospital admission and death from first-ever SARS-CoV-2 infection by age group during the Delta and Omicron periods in British Columbia, Canada
Abstract
Background: Population-based cross-sectional serosurveys within the Lower Mainland, British Columbia, Canada, showed about 10%, 40% and 60% of residents were infected with SARS-CoV-2 by the sixth (September 2021), seventh (March 2022) and eighth (July 2022) serosurveys. We conducted the ninth (December 2022) and tenth (July 2023) serosurveys and sought to assess risk of severe outcomes from a first-ever SARS-CoV-2 infection during intersurvey periods.
Methods: Using increments in cumulative infection-induced seroprevalence, population census, discharge abstract and vital statistics data sets, we estimated infection hospitalization and fatality ratios (IHRs and IFRs) by age and sex for the sixth to seventh (Delta/Omicron-BA.1), seventh to eighth (Omicron-BA.2/BA.5) and eighth to ninth (Omicron-BA.5/BQ.1) intersurvey periods. As derived, IHR and IFR estimates represent the risk of severe outcome from a first-ever SARS-CoV-2 infection acquired during the specified intersurvey period.
Results: The cumulative infection-induced seroprevalence was 74% by December 2022 and 79% by July 2023, exceeding 80% among adults younger than 50 years but remaining less than 60% among those aged 80 years and older. Period-specific IHR and IFR estimates were consistently less than 0.3% and 0.1% overall. By age group, IHR and IFR estimates were less than 1.0% and up to 0.1%, respectively, except among adults aged 70–79 years during the sixth to seventh intersurvey period (IHR 3.3% and IFR 1.0%) and among those aged 80 years and older during all periods (IHR 4.7%, 2.2% and 3.5%; IFR 3.3%, 0.6% and 1.3% during the sixth to seventh, seventh to eighth and eighth to ninth periods, respectively). The risk of severe outcome followed a J-shaped age pattern. During the eighth to ninth period, we estimated about 1 hospital admission for COVID-19 per 300 newly infected children younger than 5 years versus about 1 per 30 newly infected adults aged 80 years and older, with no deaths from COVID-19 among children but about 1 death per 80 newly infected adults aged 80 years and older during that period.
Interpretation: By July 2023, we estimated about 80% of residents in the Lower Mainland, BC, had been infected with SARS-CoV-2 overall, with low risk of hospital admission or death; about 40% of the oldest adults, however, remained uninfected and at highest risk of a severe outcome. First infections among older adults may still contribute substantial burden from COVID-19, reinforcing the need to continue to prioritize this age group for vaccination and to consider them in health care system planning.
Accumulating evidence indicates that people with a history of both vaccination against SARS-CoV-2 and SARS-CoV-2 infection are at lower risk of severe outcomes from COVID-19 than those with neither or either exposure alone.1–5 Even in the context of high vaccine coverage, understanding the residual fraction by age that remains uninfected is important to ongoing risk assessment. Early in the pandemic, older age and male sex were identified as independent predictors of severe COVID-19,6–8 but surveillance-based estimates of per case risk of hospital admission or fatality may be skewed by differential health care–seeking behaviours, testing and case finding. Seroprevalence estimates enable better capture and quantification of infections,9 but their interpretation and generalizability depend on the source population (e.g., blood donors, prenatal screening), which can sometimes exclude relevant groups of the population (e.g., young children, older adults, males).
Between March 2020 and August 2022, the British Columbia Centre for Disease Control (BCCDC) conducted 8 cross-sectional, population-based SARS-CoV-2 serosurveys using a longstanding protocol that was first developed for emerging and pandemic influenza risk assessment.9–15 Sampling included people of both sexes and all age groups ( 80 yr) residing in the Lower Mainland, BC. By the sixth (September 2021, mid-Delta wave), seventh (March 2022, following the winter 2021–2022 Omicron epidemic) and eighth (July 2022, Omicron) serosurveys, about 10%, 40% and 60%, respectively, had serological evidence of SARS-CoV-2 infection (Figure 1 and Appendix 1, Supplementary Figure 1, available at www.cmaj.ca/lookup/doi/10.1503/cmaj.230721/tab-related-content). 9,16,17 Although at least 70% of children and young adults had been infected by the end of the 8 serosurveys, more than half of adults older than 60 years remained uninfected.9 To understand subsequent changes in infection-induced seroprevalence, notably among older adults, we conducted ninth and tenth serosurveys in December 2022 and July 2023, respectively. Using data on cumulative infection-induced seroprevalence, population census and severe outcomes, we sought to estimate age- and sex-specific hospitalization and fatality ratios (IHRs and IFRs) of first-ever SARS-CoV-2 infection during specified intersurvey periods.
” data-icon-position data-hide-link-title=”0″>
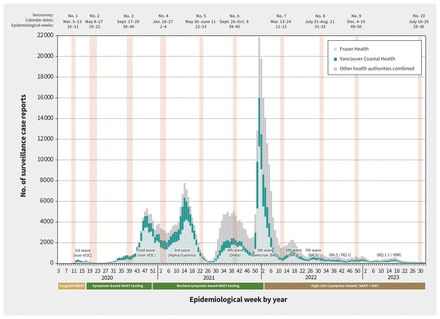
Provincial surveillance case reports by epidemiological week and timing of serosurveys, British Columbia, Canada, January 2020 (epidemiological week 3) to August 2023 (epidemiological week 32). Weekly surveillance case reports of SARS-CoV-2 infections confirmed by nucleic acid amplification test (NAAT) were reported to the British Columbia Centre for Disease Control (BCCDC) from the Fraser Health Authority (HA) and Vancouver Coastal HA in the Lower Mainland, as well as other provincial HAs (combined).17 Case tallies are grouped by epidemiological week (7-d period) as per standard surveillance methods for comparing data by period from year to year. Serosurveys exclude those identified as assisted living, independent living or long-term care facility residents but provincial case tallies do not apply those exclusions. Epidemic waves are indicated with the predominant variant of concern (VOC).16 Changes in publicly funded access to NAATs or rapid antigen tests (RATs) are displayed below the x-axis.9
Methods
Study design and sampling
Figure 1 shows the timing of all serosurveys, overlaid on surveillance case reports of SARS-CoV-2 infections (confirmed by nucleic acid amplification test [NAAT]).9,16,17 Table 1 provides details pertaining to the sixth to tenth serosurveys that we used in the current analyses. The source population was patients presenting for bloodwork to a LifeLabs diagnostic service centre, the only outpatient laboratory network serving residents of the Lower Mainland, which includes the Fraser and Vancouver Coastal Health Authorities.9 Under legal order of the Provincial Health Officer (B.H.), LifeLabs provided BCCDC investigators with a convenience sample of 2000 anonymized, residual sera from Lower Mainland residents collected during the designated serosurvey period, including 200 samples per age group (0–4 yr, 5–9 yr and by 10-yr category through ≥ 80 yr), with equal numbers by sex. Specimens collected for SARS-CoV-2 testing and those from long-term care, assisted living or prison residents were excluded. Stored residual sera collected during the designated serosurvey period were pulled concurrently and consecutively by the LifeLabs central processing centre until age- and sex-specific quotas were met.
Timing of SARS-CoV-2 serosurveys contributing to cumulative and period-specific seroprevalence estimation, Lower Mainland, British Columbia, Canada
Seroprevalence estimation
Detection of SARS-CoV-2 antibody was based on commercial chemiluminescent immunoassays to detect anti-spike (S1) or anti-nucleocapsid (NP) antibody (Table 1).9,18–25 In a previous publication of the sixth to eighth serosurveys, we applied 3 chemiluminescent immunoassays per serosurvey and defined seroprevalence by dual-assay positivity, interpreted orthogonally.9 Orthogonal approaches were initially required in the context of low seroprevalence to address specificity issues (i.e., to minimize false positives); those concerns are less important in the context of high seroprevalence.26–28 We therefore used nonorthogonal testing for the ninth and tenth serosurveys and, for consistency, similarly reanalyzed findings from the sixth to eighth serosurveys. We used the findings of 2 chemiluminescent immunoassays per serosurvey, omitting findings of the third (anti-S1) assay previously applied during sixth to eighth serosurveys (Table 1).9 We defined infection-induced seropositivity by detection of anti-NP. We defined any seropositivity (vaccine- or infection-induced) by detection of anti-NP, anti-S1 or both (Table 1). We estimated seroprevalence with 95% credible intervals (CrIs) by Bayesian analysis, adjusting for age, sex and health authority, with median summaries of the posterior presented (rather than the mean, as in our previous publication) to address the potential for extreme values (Appendix 1, Supplementary Material 1).9,29–31
Age- and period-specific risks of severe outcomes
As detailed in Appendix 1, Supplementary Material 1, we estimated the number of first SARS-CoV-2 infections based on the intersurvey difference in cumulative infection-induced seroprevalence, representing the fraction of the whole population acquiring a first-ever infection during the specified intersurvey period. We simulated first infection risks from a binomial distribution by age, sex, health authority and intersurvey period, with Bayesian-adjusted median estimates applied to 2022 estimates of the Lower Mainland population to generate the number of first infections.32 We censored negative intersurvey risks of first infection as implausible. We aggregated results by age and period, and to further explore risk estimates by sex, we aggregated at the level of age, sex and period.
The severe outcomes we studied were hospital admissions for COVID-19 and deaths from COVID-19. We tallied severe outcomes across intersurvey periods, which spanned the period from the beginning of 1 serosurvey to the end of the complete epidemiological week of a referent date 2 weeks before the last serum collection date of the next serosurvey, accounting for the typical 10–14-day span of serum collection and comparable lag to antibody development (Table 1).9 We extracted data on severe outcomes from the BC COVID-19 Cohort (BCC19C), a public health surveillance platform that integrates various administrative data sets, including the discharge abstract database (DAD) for hospital admissions,33 the provincial vital statistics database for deaths34 and the BCCDC integrated COVID-19 case surveillance data for notifiable (NAAT-confirmed) case reports (Appendix 1, Supplementary Table 1). We extracted all data on Aug. 24, 2023. We could not estimate IHR and IFR for the ninth to tenth intersurvey period because of incomplete data on hospital admissions and deaths.
We restricted hospital admissions for COVID-19 in the BCC19C to acute care admissions among Lower Mainland residents for whom the main DAD diagnostic field was specified as codes U07.1 or U07.3 (i.e., due to virologically confirmed COVID-19 or multi-inflammatory syndrome), from the Canadian version of the International Classification of Diseases and Related Health Problems, 10th Revision (ICD-10-CA); we similarly restricted deaths by underlying cause in the vital statistics data set.33–38 To correspond with the denominator of first-ever infections, we excluded people admitted to hospital for COVID-19 with codes U07.1 or U07.3 in a previous DAD record since Jan. 1, 2020, or who had a NAAT-positive specimen collected 90 days or more before admission or death (potential reinfections) identified through patient master key linkage with the BCC19C’s integrated case surveillance data set (Appendix 1, Supplementary Table 1).
We derived period-specific IHR and IFR percentages with 95% CrIs by age group and sex as the tally of hospital admissions and deaths because of COVID-19 divided by SARS-CoV-2 infections. As derived, IHR and IFR estimates represent the risk of severe outcomes from a first-ever SARS-CoV-2 infection acquired during the specified intersurvey period. In addition to the sampled age groups, we explored other categorizations; we omitted infants younger than 1 year (considering maternal antibody) and substratified adults aged 60–69 years as 60–64 years and 65–69 years, given that Canadian vaccine recommendations emphasize people aged 65 years and older.39 We also explored the effect of not censoring negative infection likelihoods, and of not excluding hospital admissions or deaths that may have been reinfections.
Ethics approval
The study was approved by the University of British Columbia Clinical Research Ethic Board (H20-00653). Analyses of severe outcomes were undertaken under the BCCDC population health surveillance and risk assessment mandate, with review waiver provided by the University of British Columbia Clinical Research Ethic Board.
Results
Population and participant profiles
We describe provincial vaccine availability, deployment and coverage in Appendix 1, Supplementary Material 2.40 Overall, 1- and 2-dose vaccine coverage was already high by the sixth serosurvey at about 80% and 75%, respectively. This varied by age, with more than 95% of adults aged 70 years and older vaccinated twice.
Of 2000 participant serum samples collected during each of the ninth (December 2022) and tenth (July 2023) serosurveys, 1374 (69%) and 1332 (67%), respectively, were from Fraser Health Authority residents, which is comparable to previous serosurveys,9 the distribution within the Lower Mainland source population (61%)32 and reported cases of NAAT-confirmed SARS-CoV-2 infection within surveillance data (68%).17 Participant median age (39.5 yr) and sex (50% female) were also representative of the Lower Mainland source population (Table 2 and Appendix 1, Supplementary Table 5 and Supplementary Table 6).9,32
SARS-CoV-2 cumulative seroprevalence estimates, Lower Mainland, British Columbia, Canada
Cumulative seroprevalence estimates
We show crude tallies and cumulative seroprevalence estimates based on nonorthogonal analysis of the sixth to tenth serosurveys in Appendix 1, Supplementary Table 7. Compared with previous orthogonal analysis,9 the absolute difference in overall and age-specific Bayesian-adjusted estimates of cumulative infection-induced seroprevalence was less than 2% absolute, with most differing less than 0.5% (Figure 2 and Table 2).
” data-icon-position data-hide-link-title=”0″>

Cumulative vaccine- and infection-induced SARS-CoV-2 seroprevalence by age group, sixth to tenth serosurveys, in the Lower Mainland, British Columbia, Canada (September 2021–July 2023). (A) Side-by-side comparison of the sixth to ninth serosurveys to illustrate seroprevalence progression. (B) Ninth and tenth serosurveys, presented separately for comparison of recent age-related patterns. Detailed findings are provided in Table 2. Darker bars indicate infection-induced seroprevalence. Lighter bars, combined with the darker bars, indicate overall (vaccine-induced, infection-induced or both) seroprevalence. Infection-induced estimates were defined by anti-nucleocapsid positivity. Overall estimates were defined by anti-spike or anti-nucleocapsid positivity. Estimates were based on Bayesian analyses, standardized for age, sex and health authority. Estimates from the sixth to eighth serosurveys are updated from our previous study,9 consistently applying the same nonorthogonal approach. Note: CrI = credible interval.
By the ninth serosurvey (December 2022), cumulative infection- induced seroprevalence reached 74% overall; seroprevalence was highest (> 80%) among people younger than 30 years, decreasing thereafter by 10-year age group, and lowest (< 50%) among adults aged 80 years and older (Figure 2 and Table 2). Estimates increased only slightly by the tenth serosurvey (July 2023) to 79% overall, with the highest seroprevalence (> 80%) seen in all age groups younger than 50 years, decreasing by 10-year age group thereafter, and the lowest seroprevalence (< 60%) seen among adults aged 80 years and older (Figure 2 and Table 2). Seroprevalence did not meaningfully differ when we simultaneously stratified by age group and by health authority or sex (Appendix 1, Supplementary Table 5 and Supplementary Table 6). Seroprevalence estimates did not meaningfully differ with further age substratification of those aged 60–69 years nor among children younger than 5 years with exclusion of infants younger than 1 year (Appendix 1, Supplementary Table 8).
Period-specific severe outcome risks
Period-specific changes in cumulative infection-induced seroprevalence and estimated first infections are displayed in Appendix 1, Supplementary Table 9. Figure 3 shows a flowchart of included hospital admissions with their distribution by age group and period shown in Table 3.
” data-icon-position data-hide-link-title=”0″>

Flowchart of hospital admissions for COVID-19 attributed to first-ever SARS-CoV-2 infection. We used the British Columbia COVID-19 Cohort. All data were extracted on Aug. 24, 2023. Where step-specific tallies of U07.1- and U07.3-coded hospital admissions do not sum to the displayed total, it is because both diagnostic codes were specified. In addition, but not displayed here, of 1346 deaths identified within the vital statistics database since Jan. 1, 2020, among Lower Mainland residents with underlying cause specified as U07.1 (none specified as U07.3) during the span of intersurvey periods, we excluded 59 (4.4%) with a SARS-CoV-2 NAAT-positive test 90 days or more before date of death. Fewer than 1% of hospital admissions identified provincially were missing information to assign health authority to the Lower Mainland. We did not exclude any hospital admissions or deaths on the basis of missing age or date of admission or death. We did not exclude any hospital admissions on the basis of missing sex, and excluded fewer than 10 fatalities on this basis, handled as indicated in Appendix 1, Supplementary Material 1. Note: FHA = Fraser Health Authority, NAAT = nucleic acid amplification test, VCHA = Vancouver Coastal Health Authority.
Estimated period-specific risk of hospital admission and death from first-ever SARS-CoV-2 infection, by age group, Lower Mainland, British Columbia, Canada
Estimates of IHR and IFR in the 3 periods studied were consistently less than 0.3% and 0.1% overall. By age group, IHR and IFR estimates were less than 1% and up to 0.1%, respectively, except among adults aged 70–79 years during the sixth to seventh intersurvey period (IHR 3.3% and IFR 1.0%) and among adults aged 80 years and older during all periods (IHR 4.7%, 2.2% and 3.5%; IFR 3.3%, 0.6% and 1.3%) (Figure 4 and Table 3). The risk of severe outcomes consistently followed a J-shaped age pattern. Risks were higher among those aged 80 years and older compared with all other age groups each period except those aged 70–79 years, with whom CrIs overlapped during the sixth to seventh period.
” data-icon-position data-hide-link-title=”0″>

Estimated risk of hospital admission and death from first-ever SARS-CoV-2 infection acquired during the specified intersurvey period, by age group, Lower Mainland, British Columbia, Canada. (A) Period-specific infection hospitalization ratios (IHRs) and (B) infection fatality ratio (IFRs) by age group. Panels A and B do not show 95% credible intervals (CrIs) (but are provided in Table 3) for better resolution, given that upper CrIs extended past 10%. (C) Period-specific IHRs (log10 scale) and (D) IFRs (log10 scale) with 95% CrIs.
Among children younger than 5 years, most (60%–65%) hospital admissions for COVID-19 were among infants younger than 1 year (Appendix 1, Supplementary Table 10). With the exclusion of infants in sensitivity analyses, IHR estimates among children aged 1–4 years were consistently halved but were still higher than among those aged 5–9 years. We lacked sample size to reliably model estimates for infants, but because about one-third of admissions among those younger than 5 years occurred among those aged 1–4 years, compared with two-thirds among infants younger than 1 year, we anticipate infant IHRs could be about 8-fold higher than those aged 1–4 years. Compared with adults aged 60–64 years, the IHR and IFR estimates were somewhat higher among those aged 65–69 years; in the eighth to ninth intersurvey period, the IHR was 0.07% and the IFR was 0.01% among those aged 60–64 years, compared with 0.21% and 0.04%, respectively, among those aged 65–69 years (Appendix 1, Supplementary Table 10). By sex, we observed a consistent pattern of higher IHR and IFR estimates among males compared with females, although CrIs largely overlapped when simultaneously stratified by age and sex (Figure 5 and Appendix 1, Supplementary Tables 11–13). Finally, we observed minimal change from primary estimates in sensitivity analyses with and without censoring of negative infection likelihoods and with and without exclusion of hospital admissions and deaths potentially owing to reinfection (Appendix 1, Supplementary Table 14 and Supplementary Table 15).
” data-icon-position data-hide-link-title=”0″>

Estimated period-specific risk of hospital admission and death from first-ever SARS-CoV-2 infection by sex, overall and by age group, Lower Mainland, British Columbia, Canada. (A) Period-specific infection hospitalization ratios (IHRs) and (B) infection fatality ratio (IFRs) with their 95% credible intervals (CrIs), using log10 scale. Because of fewer severe outcomes, we did not stratify IHR and IFR estimates by sex for separate age groups younger than 50 years. All ages refers to all age groups combined from younger than 5 years to 80 years and older. Details are provided in Appendix 1, Supplementary Tables 11–13.
Interpretation
To inform population risk assessment and response to the COVID-19 pandemic, we used seroprevalence estimates and severe outcome data to derive estimates of the risk of hospital admissions and death per first-ever SARS-CoV-2 infection. By the end of the third year of the pandemic (ninth serosurvey, December 2022) and middle of the fourth year (tenth serosurvey, July 2023), at least 75% and 80%, respectively, of Lower Mainland residents showed serological evidence of a previous SARS-CoV-2 infection. Whereas more than 80% of children and adults younger than 50 years had been infected and were at low risk of hospital admission or death, nearly half of adults aged 80 years and older remained uninfected and at highest risk of severe outcome upon first infection.
Our seroprevalence findings align with a report from Canadian Blood Services, which stated that about 80% of donors nationally were infected by June 2023.41 They also found that infection rates were highest at 90% in their youngest cohort (17–24 yr) and lowest at 70% in their oldest cohort (≥ 60 yr). A recent compilation of Canadian studies similarly reported that 76% of participants had evidence of infection by March 2023.42 Our seroprevalence approach offers the advantage of serial and simultaneous sampling of both sexes across the life span, including the extremes of age, both the very young and very old, which are under-represented in other serosurvey approaches. Moreover, by combining population seroprevalence estimates and severe outcome statistics, we can directly compare their age- and sex-specific risks of severe outcomes.
As previously reported for influenza,43 we observed a J-shaped pattern of age-related risk of severe outcomes that started to increase at about 50 years of age, although risks of hospital admission and fatality from COVID-19 were consistently low at less than 1% and up to 0.1% or less, respectively, until 80 years of age (age 70–79 yr during the sixth to seventh intersurvey period). Very old adults were at highest risk, contributing half of all hospital admissions for COVID-19 and two-thirds to three-quarters of all COVID-19 deaths during the final 2 analysis periods. During the eighth to ninth intersurvey period between July and December 2022, estimated IHRs among children younger than 5 years, and adults aged 70–79 years and 80 years and older correspond with about 1 hospital admission for COVID-19 per 300, 100 and 30 newly infected people, respectively, indicating at least a 3- to 10-fold higher risk for the oldest adults. During the same period, no child or young adult died, whereas estimated IFRs correspond with about 1 COVID-19 death per 900 newly infected adults aged 70–79 years and about 1 per 80 newly infected adults aged 80 years and older, indicating a risk at least 10-fold higher for the latter group. Earlier seroprevalence-based studies documented higher risk for males,44–47 notably older males.46,47 In our study, older males also tended to be at highest risk of severe outcome, although CrIs largely overlapped when analyses were stratified by both age and sex.
Our estimates of the risk of hospital admission or death from a first-ever SARS-CoV-2 infection were low overall but derived in a highly vaccinated population. Risks are anticipated to be greater among unvaccinated and lower among previously infected groups of patients, with the lowest risk among those who are both vaccinated and previously infected.1–5 Before Omicron, when vaccine coverage was lower, severe outcome risks estimated by others were higher than we report here;44–53 IFRs by single-year of age were estimated to reach 1% from age 60 years, 3% from age 70 years, 8% from age 80 years and 20% from age 90 years.48 Post-Omicron, fewer seroprevalence-based estimates are available, but among Danish blood donors aged 17–72 years, more than 95% of whom were vaccinated twice, the IFR from January to March 2022 was 0.02% among the oldest adults (61–72 yr).54 Danish estimates are lower than our IFR for adults aged 60–69 years (0.11%) during our overlapping sixth to seventh intersurvey period (September 2021–March 2022), when we observed the highest period-specific risks, notably among adults aged 80 years and older (3.3%). Unlike the Danish context, however, our sixth to seventh intersurvey period spanned both Delta and Omicron circulation, with other studies showing that, independent of other variables, Delta was more severe than Omicron.55–58 Thereafter, during our seventh to eighth intersurvey period of mostly Omicron BA.2 followed by BA.5, severe outcome risks were lower but increased again among older adults during the eighth to ninth intersurvey period of mostly BA.5 followed by BQ.1. Greater severity of BA.5 infections than BA.2 infections has been noted by others and may have influenced the pattern we observed.59
Other factors may have contributed to the higher risk among older adults during the autumn of 2022. By the eighth serosurvey in July 2022, more than half of older adults had received 4 doses of mostly monovalent, ancestral SARS-CoV-2 vaccines, increasing to more than two-thirds by the ninth serosurvey in December 2022, when as many as one-third of older adults had received 5 doses (bivalent BA.1 or BA.4/5) (Appendix 1, Supplementary Material 2).40 Earlier receipt and repeat doses of monovalent or bivalent products containing antigenically distinct (e.g., original or BA.1) strains may have contributed to reduced or waning cross-protection, especially against more immune-evasive BQ.1 subvariants.60–69 The extent to which original priming and its booster reinforcement may affect response to subsequent antigenically distinct variants, and the capacity to overcome that through updated antigen or other approaches, remain under debate for both influenza and SARS-CoV-2.70,71
To date, recommendations for prioritization of updated vaccines by age for the fall of 2023 have varied, with some jurisdictions in Europe targeting people aged 60 years and older,72 and other countries, including Canada and the United Kingdom, targeting those aged 65 years and older.39,73 Our exploratory analyses showed some gradation in the risks of hospital admission and fatality per first infection between those aged 60–64 years (about 1 per 1400 and 10 000, respectively) versus 65–69 years (about 1 per 500 and 2500, respectively). The 65-year threshold aligns more closely with seasonal influenza immunization programs in most provinces.74 The greatest risk of severe outcomes from SARS-CoV-2 infection, however, was among patients aged 80 years and older. Ultimately, in addition to the risk of severe outcomes, the population impact of COVID-19 by age is also determined by the absolute size of the age cohort and the likelihood of having been, or becoming, infected.
Limitations
All serosurveys are subject to potential biases and, as previously discussed,9 our use of residual clinical specimens will have tended, on balance, to underestimate seroprevalence. The intersurvey differences in cumulative infection-induced seroprevalence we report de facto subtract previous infections, thereby representing the risk of first-ever infection as acquired during the specified period. Our serial, cross-sectional, population-based approach does not follow the same cohort of people longitudinally and cannot predict or account for antibody waning or its complex variation by, for instance, age or vaccine status. As such, our estimates are best interpreted as indicating at least that number infected between serosurveys. Risks of hospital admission and fatality per infection would then conversely be overestimates. Even though our estimates of the risk of severe outcomes were low, they are likely even lower for most of the population, particularly among those both previously vaccinated and infected who are now the majority overall.1–5 In that context, and given lower likelihood of previous infection among older adults, the overall J-shaped age pattern we reinforce is likely robust. To identify severe outcomes and assign attribution to COVID-19, we used the DAD and vital statistics databases, allowing sufficient lag (> 37 wk) to ensure completeness of data (typically requiring up to 6 mo). Although we used well-established DAD and vital statistics data sets, including validated ICD-10-CA codes for COVID-19 outcomes,33–38 we have no official standards against which to verify our data. Reassuringly, the 47% of hospital admissions we identified as for COVID-19 (Figure 3), is comparable to the proportion (45%) assigned as for (v. with) COVID-19 based on more limited chart review within the Vancouver Coastal Health Authority.75–77 The proportion we excluded (11%) on the basis of potential reinfection is similar to the proportion with multiple hospital admissions (n = 1906, 12%) among 16 333 total hospital admissions for people with a positive SARS-CoV-2 test as identified in a separate BCCDC review between Apr. 1, 2022, and Mar. 31, 2023.78 We cannot rule out some misclassification or omission, and given targeted NAAT testing, our efforts to further reduce potential reinfections among hospital admissions or deaths, could not capture those not tested. Reassuringly, our estimates did not vary with or without exclusion of identified potential reinfections. Our findings address severe acute outcomes but do not take into account longer-term post-COVID-19 conditions, nor did we factor reasons for or durations of admission, which likely vary by age. Finally, we did not have data on ethnicity, socioeconomic factors or comorbidities, and our use of sex as specified within available data sets may or may not represent people’s self-identified gender.
Conclusion
By July 2023, around 80% of Lower Mainland, BC, serosurvey participants had been infected with SARS-CoV-2. In the context of high vaccine coverage contributing to hybrid protection, most children and adults were at low risk of severe outcomes from COVID-19. A substantial proportion (> 40%) of the oldest adults, however, remained uninfected and at highest risk of hospital admission or death. First-ever SARS-CoV-2 infections among older adults may still contribute substantial COVID-19 burden, reinforcing the importance of their continued prioritization for vaccination and their consideration in health care system planning.
Acknowledgements
The authors wish to acknowledge the serological testing oversight and contribution of the British Columbia Centre for Disease Control (BCCDC) Public Health Laboratory and Providence Health Care Special Chemistry Laboratory, including Julia Dyer, Tamara Pidduck, Jesse Kustra, Mandana Shamlou, Laura Burns and Marc Kour. They thank Rhonda Creswell and Iva Tong of LifeLabs for supervision of serum collection. They also thank Hind Sbihi, Brent Gabel, Solmaz Setayeshgar, Yayuk Joffres, Hannah Caird, Mieke Fraser, Sharon Relova, Jimmy Lopez, Joy Ding and Ayisha Khalid of the BCCDC for their supportive input, consultation or analyses. Finally, they thank the many other frontline, regional and provincial practitioners, including clinical and public health providers, epidemiologists, Medical Health Officers and others for their contributions to surveillance case reporting in British Columbia.
Footnotes
-
Competing interests: Danuta Skowronski reports grants from the Canadian Institutes of Health Research and the BCCDC Foundation for Public Health, paid to her institution, for other SARS-CoV-2 work. Romina Reyes is chair of the British Columbia Diagnostic Accreditation Program committee. As the Provincial Health Officer with authority under the emergency provisions of the Public Health Act, Bonnie Henry authorized the provision and analysis of the anonymized sera used in this study; the study was separately reviewed and approved by the University of British Columbia Clinical Research Ethics Board. No other competing interests were declared.
-
This article has been peer reviewed.
-
Contributors: Danuta Skowronski, Samantha Kaweski, Michael Irvine and Kate Smolina conceived and designed the study. Samantha Kaweski, Shinhye Kim, Suzana Sabaiduc, Romina Reyes, Bonnie Henry and Inna Sekirov acquired data. Danuta Skowronski, Samantha Kaweski, Michael Irvine and Erica Chuang analyzed data. Danuta Skowronski, Samantha Kaweski and Michael Irvine drafted the manuscript. All of the authors revised it critically for important intellectual content, gave final approval of the version to be published and agreed to be accountable for all aspects of the work.
-
Funding: Funding was provided in part by the Public Health Agency of Canada (no. 2021-HQ-000067) and the Michael Smith Foundation for Health Research (no. 18934).
-
Data sharing: Aggregate serological data are provided within the manuscript and the supplementary material. Any further data sharing of seroprevalence data will be considered upon reasonable request to the corresponding author with appropriate review and aggregation, as required to comply with the provincial legislation under which the data were assembled and respecting privacy and confidentiality requirements. Severe outcome and integrated case surveillance data sets used for hospital admission and fatality estimates were accessed through the British Columbia COVID-19 Cohort (BCC19C), a public health surveillance platform integrating COVID-19 datasets with administrative data holdings for the BC population. The BCC19C was established and is maintained through operational support from Data Analytics, Reporting and Evaluation (DARE) and the BC Centre for Disease Control (BCCDC) at the Provincial Health Services Authority. The authors are not permitted to share these data; BCC19C data are only available to researchers who request and meet the criteria for access.
-
Disclaimer: The views expressed herein do not necessarily represent the views of the Public Health Agency of Canada. All inferences, opinions and conclusions drawn in this manuscript are those of the authors and do not reflect the opinions or policies of the Data Steward(s).
- Accepted September 20, 2023.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) licence, which permits use, distribution and reproduction in any medium, provided that the original publication is properly cited, the use is noncommercial (i.e., research or educational use), and no modifications or adaptations are made. See: https://creativecommons.org/licenses/by-nc-nd/4.0/