Cancer and neoplasms
Successful treatment of concurrent FL and TNBC
Zhou Zhu,1,2,* Na Zhou,1,* Shuangni Yu,3 Xin Gao,4 Xin Cheng,5 Yingyi Wang,1 Chunmei Bai1
1Department of Medical Oncology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, People’s Republic of China; 2 4+4 Medical Doctor Program, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, People’s Republic of China; 3The Department of Pathology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, People’s Republic of China; 4The Department of Radiology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, People’s Republic of China; 5The Department of Nuclear Medicine, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, People’s Republic of China
Correspondence: Yingyi Wang, Department of Medical Oncology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, People’s Republic of China, Email [email protected]
Background: Co-occurrence of breast cancer and non-Hodgkin’s lymphoma is a rare condition with diagnostic and therapeutic challenges. The coexistence of follicular lymphoma (FL) and triple-negative breast cancer (TNBC) has not been described previously.
Case Presentation: A 46-year-old woman, already suffering a history of untreated, advanced-stage, high tumor burden FL, was admitted for a rapidly progressing right breast mass. Ultrasonography showed an 8.3 × 3.6 × 4.1 cm fungating mass in the right breast with enlarged lymph nodes (LNs) in bilateral axillae. PET-CT demonstrated increased 18F- FDG activity in right breast mass, LNs on both sides of the diaphragm, enlarged spleen, and bone marrow. Biopsy of the right breast mass revealed TNBC. The patient underwent neoadjuvant therapy with R-CHOP and achieved partial response of breast tumor. However, TNBC progressed after three cycles of R-CHOP. According to the next-generation sequencing (NGS) assay on breast mass showing a homologous recombination repair (HRR) deficiency (HRD) score of 72, the neoadjuvant regimen was changed to rituximab plus nab-paclitaxel and cisplatin (R-TP) and resulted in significant tumor regression. The patient then underwent right mastectomy with an axillary LN dissection. After the surgery, she was regularly monitored and given adjuvant therapy with R-TP and radiotherapy.
Conclusion: The coexistence of FL and HRD-positive TNBC poses diagnostic and treatment challenges. Well-founded neoadjuvant strategy based on multidisciplinary team (MDT) discussion and NGS warranted a good outcome in this case.
Keywords: multiple primary malignancies, follicular lymphoma, triple-negative breast cancer, HRD, neoadjuvant therapy, MDT, case report
Introduction
Cancer survivors are at increased risk of second primary malignancies. Multiple primary malignancies (MPMs) occur in 0.4–18.4% of cancer cases.1,2 However, metachronous or synchronous presentation of breast cancer (BC) and follicular lymphoma (FL) is rare.3–5 It is not clear whether these two neoplasms occur by mutually promoting mechanisms and the consensus is lacking on how to treat patients with co-existing BC and FL since different stages of each disease predict diverse prognosis and demand utterly different kinds of treatment. Treatment decisions should be made by a multidisciplinary team (MDT) after the individual prognoses of both malignancies have been scrupulously weighed, and a win–win situation has been negotiated.
Triple-negative breast cancer (TNBC), representing 10–15% of BCs4, is the most aggressive subtype with abysmal outcomes. Currently, treatment modalities for TNBC without distant metastasis include surgery, chemotherapy, and radiology. Neoadjuvant chemotherapy was indicated for locally advanced (T1c or above) or limited clinically node-positive TNBC patients with the intent to downstage the disease as well as evaluate the effectiveness of systemic therapy.6 The addition of platinum to traditional neoadjuvant regimens was proved to be able to further improve the responses and prolong the survival of patients with TNBC, especially in those who harbor homologous recombination repair (HRR) deficiency (HRD).7,8
In the present study, we report the first case of TNBC coexistent with high tumor burden FL, successfully treated with neoadjuvant target therapy combined with chemotherapy and make a review of relevant literature.
Case Presentation
A 46-year-old woman presented to our clinic with a right palpable breast mass in January 2022. Ultrasonography (US) revealed a 2.3 × 1.7 × 1.3 cm hypoechoic mass in the right breast at 11 o’clock position, with a BI-RADS category of 4a. Multiple enlarged lymph nodes (LNs) were detected in bilateral axillae. Her past medical history was notable for a low-grade FL under watchful waiting (Figure 1). A retroperitoneal mass was found during a regular health examination in December 2020 and biopsy suggested Grade 1–2 FL. Immunohistochemistry (IHC) revealed positive expression of CD21 (FDC), CD20, CD23, CD10, BCL-2 and BCL-6, as well as negative expression of cyclin D1. Ki-67 index was 20%. A positron emission tomography (PET)-computerized tomography (CT) scan demonstrated significant uptake (SUVmax 7.6) in the retroperitoneal LN, measuring 4.1 × 2.9 cm. Bilateral axillary, left parasternal, paravertebral, intraabdominal, intrapelvic, and bilateral inguinal LNs were also 18F-fluorodeoxyglucose (FDG)-avid. She remained in good performance status and asymptomatic under close surveillance by her hematologist for over a year.
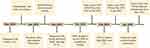 |
Figure 1 Timeline of this case report. Abbreviations: FL, follicular lymphoma; HRD, homologous recombination deficiency; MDT, multidisciplinary team; TNBC, triple-negative breast cancer. |
The patient was immediately suggested an open biopsy or core biopsy to determine the nature of the right breast mass. However, she refused due to personal reasons. In October 2022, the patient presented with a large mass in her right breast with skin involvement. She also suffered from recurrent fevers with headache, chills, and joint pain since August 2022, followed by short of breath and rapid progression of the right breast mass. US showed an 8.3 × 3.6 × 4.1 cm mass in the right breast, with lymphadenopathy at all three levels of bilateral axillae. Whole-body PET-CT demonstrated a hypermetabolic mass in the right breast and hypermetabolic LNs on both sides of the diaphragm. Increased 18F-FDG activity was also detected in enlarged spleen and bone marrow (Figure 2A). Biopsy of the right breast mass was then performed and the pathological examination revealed no special type, histological grade 3 TNBC with a Ki-67 index of 70% (Figure 3A–D). Blood test revealed significantly high white blood cell (WBC 114.28×109/L) and lymphocyte counts (LY 106.73×109/L), low hemoglobin (HGB 97g/L), and an elevated lactate dehydrogenase (LDH) of 443 U/L. Abnormal serum tumor markers including CA19-9 of 189.2 U/mL, CA125 of 938.0 U/mL, and CA15-3 of 183.5 U/mL were also detected. Bone marrow aspiration and biopsy confirmed bone marrow infiltration by FL (Figure 3K and L). Therefore, stage IV FL with a FLIPI score of 4 and locally advanced TNBC were confirmed and both were indicated for systemic therapy.
|
Figure 2 Whole-body PET-CT before (A) and after (B) neoadjuvant therapy. After treatment, the right breast mass (red arrows) significantly decreased in size. Bilateral cervical (Orange arrows), bilateral axillary (green arrows), mediastinal (purple arrows), cardiophrenic (yellow arrows), intraabdominal, intrapelvic and bilateral inguinal (black arrows) LNs decreased in both size and 18F-FDG uptake. A significant decrease in size and 18F-FDG uptake was also detected in spleen (blue arrows). |
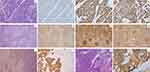 |
Figure 3 Histopathological and immunostaining examination of TNBC and FL. (A) Hematoxylin and eosin (H&E) staining of right breast specimen showing invasive ductal carcinoma. Magnification, × 400. Right breast specimen immunostaining negative for HER-2 (B), positive for P53 (C) and with a Ki-67 index of 70% (D). Magnification, × 400. (E) H&E staining of right axillary lymph nodes suggesting lymphoma infiltration. Magnification, × 100. Lymphoid follicles immunostaining positive for CD20 (F), CD10 (G), and BCL-2 (H), suggesting FL. Magnification, × 100. H&E staining (I) and AE1/AE3 immunostaining (J) of right axillary lymph node suggesting TNBC metastases. Magnification, × 100. (K) H&E staining of bone marrow specimen showing FL infiltration. Magnification, × 400. (L) Bone marrow specimen immunostaining positive for CD20. Magnification, × 400. |
The patient subsequently underwent neoadjuvant therapy with R-CHOP (rituximab, cyclophosphamide, vincristine, doxorubicin, and prednisone). After two cycles of R-CHOP, the right breast mass decreased from 8.5 × 6.7 cm to 4.9 × 5.6 cm based on CT examination (Figure 4A, B, E and F), showing a partial response (PR) according to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1.9 WBC, LY, and LDH decreased to normal limits. CA15-3 descended to 99.3 U/mL. However, the mass in the right breast increased rapidly after the completion of the third cycle of R-CHOP (Figure 4C and G). Next-generation sequencing (NGS) performed on the breast tumor sample revealed a HRD score of 72 and pathogenic mutations in ARID1A, FBXW7, SLX4, and TP53 genes. According to the NGS results, the neoadjuvant regimen was changed to R-TP (rituximab, nab-paclitaxel, and cisplatin). The patient responded well to the R-TP regimen with a rapid reduction in tumor size, from 7.4 × 8.7 cm to 3.9 × 3.7 cm (Figure 4D and H). After three cycles of R-TP, CA15-3 descended to 51.8 U/mL. PET-CT was performed again and showed that hypermetabolic LNs on both sides of the diaphragm decreased in size as well as 18F-FDG uptake. A significant decrease in size and 18F-FDG uptake was also detected in spleen (Figure 2B).
 |
Figure 4 Axial CT of the breast (red arrow) before neoadjuvant therapy (A). The right breast mass decreased from 8.5×6.7 cm to 4.9×5.6 cm (red arrow) after two-cycle of R-CHOP (B), rapidly increased to 7.4×8.7 cm (red arrow) after three-cycle of R-CHOP (C), and decreased to 3.9×3.7 cm (red arrow) after R-TP (D). The corresponding photos of the right breast mass before neoadjuvant therapy (E), after two-cycle of R-CHOP (F), after three-cycle of R-CHOP (G), and after R-TP (H). |
The patient then underwent right modified radical mastectomy and right axillary LN dissection. The pathological examination of right axillary LNs demonstrated both LN metastases from TNBC (1/50) and FL infiltration (49/50) (Figure 3E–J). The pathological staging of TNBC was T3N1M0, IIIA. After surgery, the patient continued to receive R-TP for 3 cycles and adjuvant radiotherapy for breast cancer.
Discussion
The development of breast cancer after treatment for Hodgkin’s lymphoma is a recognized occurrence, especially in female patients who have undergone chemoradiotherapy.10 However, the coexistence of breast cancer and NHL is rare and makes the clinical diagnoses and treatment quite challenging.5 A total of 17 cases of BC coexistent with FL, including the present case, have been reported in the literature. A summary of reported data is listed in Table 1. In the present case, the patient was diagnosed with dual presentation of treatment-naïve stage IV FL and HRD-positive locally advanced TNBC. Notably, this case was the only one that FL was detected prior to BC and the only one with TNBC.
|
Table 1 Double Presentation of Breast Cancer and FL |
It is not clear whether there is a direct relationship between TNBC and FL according to the present case. Theoretically, the coexisting lymphoma could prepare the ground by weakening the immune system and disabling immune cell-mediated anti-tumor pathways, which might trigger the development of a secondary carcinoma.5,20 Given that FL occurred first and received no treatment before the detection of TNBC, the prior lymphoma might be a possible cause of the coexisting BC. However, further evidence is needed to elucidate the underlying mechanisms.
The coexistence of TNBC and FL poses a diagnostic challenge. Lymphadenopathy is a common presentation of lymphoma. However, BC may also present with enlarged axillary LNs in addition to primary breast masses. Physical examination and imaging tests, including US and PET‑CT are not capable of differentiating the origin of adenopathy in such occasion. Core biopsy or excisional biopsy of axillary LNs, followed by histology and IHC staining are the exact methods to assess axillary LNs. According to the literature review, FL was diagnosed by excisional biopsy of axillary LNs in majority of the reported cases (Table 1). In the present case, axillary dissection and IHC staining also performed to make accurate staging of both FL and TNBC. In addition, regular follow-up and systematic examination of cancer patients are necessary to improve detection rate of coexistent malignancies.
Unlike those previously reported cases in which most BCs were treated with surgery followed by adjuvant therapy, while early-stage FLs were merely followed up (Table 1), both malignancies in this case progressed rapidly and come to the point where systemic treatment is imminently needed. The top priority is to stabilize both the skin-involved, large TNBC and the bone marrow involved, disseminated FL as soon as possible while to look for an opportunity for mastectomy as the systemic treatment goes. R-CHOP, a standard first-line regimen for FL, was given to the patient as the upfront treatment by MDT since the combination of doxorubicin plus cyclophosphamide in this regimen was also likely to be effective in treatment-naive TNBC. Although our original assumption has been verified, the primary BC presented early resistance to the regimen. And the nonsense mutation in FBXW7 gene identified by NGS might be the possible explanation. By introducing termination codon, the mutation would lead to early truncation of the protein, and the loss of FBXW7 has been shown to reduce the sensitivity to doxorubicin in tumor cells.21
NGS genotyping also provides information about genomic alterations that can guide the precision medicine. The switch of neoadjuvant therapy to nab-paclitaxel and platinum in this case is based on not just the simple thought that the incorporation of platinum may amplify the efficacy of neoadjuvant therapy in TNBC while benefit FL, but also the NGS results of high HRD score and mutations of multiple HRR genes (ARID1A and SLX4), which indicate the deficiency in DNA double-strand break repair and the sensitivity of tumor cells to platinum.22 Besides, poly (ADP-ribose) polymerase inhibitors (PARPi) had been proved to be able to bring clinical benefits for BC patients carrying HRR mutations beyond BRCA.23 HRD-positive TNBC is also sensitive to immune checkpoint blockade (ICB) due to a higher tumor mutation burden,24 so is FL. ICB with PARPi might be considered as salvage therapy after the progression of whichever malignancy. The biggest challenge of this case is to build a well-founded treatment strategy rather than follow the routine.
Conclusion
To the best of our knowledge, this is the first case of locally advanced TNBC coexisting with advanced-stage FL. Its management is discussed with a review of relevant literature. Decisive implement and revision of neoadjuvant therapy based on MDT discussion and NGS warranted a good outcome in this case.
Data Sharing Statement
All inquiries can be directed to the corresponding authors.
Ethics Statement and Consent for Publication
Institutional approval was not required to publish the case details. The patient provided written informed consent to the publication of this case report and any accompanying images.
Acknowledgments
The authors thank to the patient and her family for their support of this study.
Funding
This study was supported by National High Level Hospital Clinical Research Funding (2022-PUMCH-A-213, 2022-PUMCH-A-215).
Disclosure
The authors have no conflict of interest.
References
1. Weir HK, Johnson CJ, Thompson TD. The effect of multiple primary rules on population-based cancer survival. Cancer Causes Control. 2013;24(6):1231–1242. doi:10.1007/s10552-013-0203-3
2. Rosso S, De Angelis R, Ciccolallo L, et al. Multiple tumours in survival estimates. Eur J Cancer. 2009;45(6):1080–1094. doi:10.1016/j.ejca.2008.11.030
3. Hiraoka E, Masumoto N, Furukawa T, et al. Follicular lymphoma without lymphadenopathy incidentally diagnosed by sentinel lymph node biopsy during breast cancer surgery: a case report. Surg Case Rep. 2022;8(1):167. doi:10.1186/s40792-022-01524-4
4. Eto R, Nakamura R, Yamamoto N, et al. Synchronous early-stage breast cancer and axillary follicular lymphoma diagnosed by core needle biopsy: a case report. Mol Clin Oncol. 2022;16(1):3. doi:10.3892/mco.2021.2436
5. Saleem T, Mi K, Pathak R, Yari K, Lu K. Concurrent breast carcinoma and follicular lymphoma: a case series. Am J Case Rep. 2021;22:e931772. doi:10.12659/AJCR.931772
6. Liedtke C, Mazouni C, Hess KR, et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol. 2008;26(8):1275–1281. doi:10.1200/JCO.2007.14.4147
7. Sikov WM, Berry DA, Perou CM, et al. Impact of the addition of carboplatin and/or bevacizumab to neoadjuvant once-per-week paclitaxel followed by dose-dense doxorubicin and cyclophosphamide on pathologic complete response rates in stage II to III triple-negative breast cancer: CALGB 40603 (Alliance). J Clin Oncol. 2015;33(1):13–21. doi:10.1200/JCO.2014.57.0572
8. Zhang L, Chen Y, Cheng M-Y, et al. Homologous recombination deficiency predicts the response to platinum-based neoadjuvant chemotherapy in early-stage triple-negative breast cancer patients: a systematic review and meta-analysis. Ther Adv Med Oncol. 2022;14:17588359221096253. doi:10.1177/17588359221096253
9. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247. doi:10.1016/j.ejca.2008.10.026
10. Tamaoki M, NIO Y, TSUBOI K, et al. A rare case of non-invasive ductal carcinoma of the breast coexisting with follicular lymphoma: a case report with a review of the literature. Oncol Lett. 2014;7(4):1001–1006. doi:10.3892/ol.2014.1885
11. Mirkheshti N, Mohebtash M. A rare case of bilateral breast lobular carcinoma coexisting with primary breast follicular lymphoma. J Community Hosp Intern Med Perspect. 2019;9(2):155–158. doi:10.1080/20009666.2019.1579611
12. Fushimi A, Kinoshita S, Kudo R, Takeyama H. Incidental discovery of follicular lymphoma by sentinel lymph node biopsy and skin-sparing mastectomy for Paget’s disease associated with invasive breast cancer. J Surg Case Rep. 2019;2019(1):rjz008. doi:10.1093/jscr/rjz008
13. Papanastasopoulos P, Merchant S, Kenny L. Follicular lymphoma in a patient with previously treated early breast cancer; the role of PET imaging and repeat tissue diagnosis. Breast J. 2015;21(6):691–692. doi:10.1111/tbj.12511
14. Michalinos A, Vassilakopoulos T, Levidou G, Korkolopoulou P, Kontos M. Multifocal bilateral breast cancer and breast follicular lymphoma: a simple coincidence? J Breast Cancer. 2015;18(3):296–300. doi:10.4048/jbc.2015.18.3.296
15. Hahm MH, Kim HJ, Shin KM, et al. Concurrent invasive ductal carcinoma of the breast and malignant follicular lymphoma, initially suspected to be metastatic breast cancer: a case report. J Breast Cancer. 2014;17(1):91–97. doi:10.4048/jbc.2014.17.1.91
16. Laudenschlager MD, Tyler KL, Geis MC, Koch MR, Graham DB. A rare case of synchronous invasive ductal carcinoma of the breast and follicular lymphoma. S D Med. 2010;63(4):123–125.
17. Cuff KE, Dettrick AJ, Chern B. Synchronous breast cancer and lymphoma: a case series and a review of the literature. J Clin Pathol. 2010;63(6):555–557. doi:10.1136/jcp.2009.069625
18. Cox J, Lunt L, Webb L. Synchronous presentation of breast carcinoma and lymphoma in the axillary nodes. Breast. 2006;15(2):246–252. doi:10.1016/j.breast.2005.06.009
19. Barranger E, Marpeau O, Uzan S, Antoine M. Axillary sentinel node involvement by breast cancer coexisting with B-cell follicular lymphoma in nonsentinel nodes. Breast J. 2005;11(3):227–228. doi:10.1111/j.1075-122X.2005.21697.x
20. Nishikawa A, Kasai H, Koyama Y, et al. Synchronous ipsilateral carcinoma of the accessory mammary gland and primary lymphoma of the breast with subsequent rectal carcinoma: report of a case. World J Surg Oncol. 2014;12(1):286. doi:10.1186/1477-7819-12-286
21. Yi X, Lou L, Wang J, Xiong J, Zhou S. Honokiol antagonizes doxorubicin resistance in human breast cancer via miR-188-5p/FBXW7/c-Myc pathway. Cancer Chemother Pharmacol. 2021;87(5):647–656. doi:10.1007/s00280-021-04238-w
22. Watkins JA, Irshad S, Grigoriadis A, Tutt AN. Genomic scars as biomarkers of homologous recombination deficiency and drug response in breast and ovarian cancers. Breast Cancer Res. 2014;16(3):211. doi:10.1186/bcr3670
23. Tung NM, Robson ME, Ventz S, et al. TBCRC 048: Phase II Study of Olaparib for Metastatic Breast Cancer and Mutations in Homologous Recombination-Related Genes. J Clin Oncol. 2020; 38(36):4274–4282. doi:10.1200/JCO.20.02151
24. Pellegrino B, Musolino A, Llop-Guevara A, et al. Homologous recombination repair deficiency and the immune response in breast cancer: a literature review. Transl Oncol. 2020;13(2):410–422. doi:10.1016/j.tranon.2019.10.010

