Cardiovascular
A ‘manganese bullet’ targeting cardiovascular disease? Research finds potential therapy for intensive lipid lowering
Cardiovascular disease (CVD) continue to rank as the top killer in the modern world. This deadly disorder often starts with the buildup of lipid deposits or plaques within the blood vessel, silently setting the stage for atherosclerosis. Rupture of these atherosclerotic plaques, however, could clot blood vessels and lead to life-threatening conditions including heart attack or stroke.
Dyslipidemia, meaning having too much “bad” or atherogenic lipids in the blood, represents the most common cause of CVDs and is present in about 50% of the adult population. Accordingly, doctors often prescribe lipid-lowering medications (e.g. statins and proprotein convertase subtilisin/Kexin type 9 (PCSK9) inhibitors) for treating CVDs. These “block-buster” drugs, however, could only stabilize but were not able to reverse or eliminate the existing atherosclerotic plaques.
In two complementary studies, one published in Nature Cell Biology and the other in Life Metabolism, researchers have found a novel approach to achieve intensive lipid lowering, which enabled the reversal of atherosclerotic plaques in murine disease models.
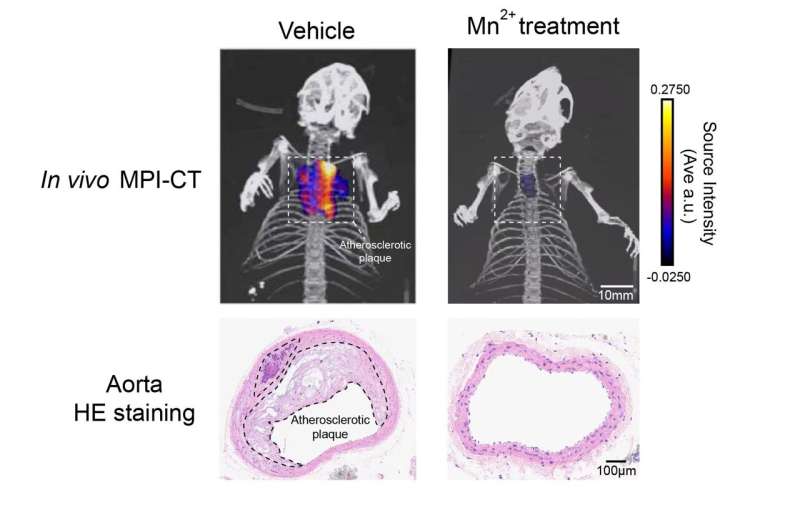
This potential therapy employs a previously unknown function of the essential element manganese. Increasing doses of extra manganese, even when conveniently provided to mice via diets, dramatically reduce blood lipids and clear out the atherosclerotic plaques that have already built up in the blood vessels.
Dr. Xiao Wang, one of the lead authors of the work, explains, “Manganese is considered as the least understood essential element, mainly playing supporting roles in enzymatic reactions. Yet, we’ve uncovered an active, signaling role of the manganese ion in controlling lipid delivery into the blood.”
Bulk lipids, including cholesterol and triglycerides, are transported into the blood via specialized carriers named lipoproteins. These lipid carriers are much larger and more complex compared to most of the other secretory factors in the blood.
The researchers discovered that these lipid-ferrying lipoproteins rely on the biomolecular condensation of a common cellular machinery, known as the coat protein complex II (COPII) complex. Moreover, COPII condensation needs to be finely balanced at the right level to support bulk lipid delivery.
After dissecting a series of highly unexpected observations, the researchers further discovered that manganese ions could directly bind COPII and enhance its condensation. This in turn alters the fine balance in COPII regulation, resulting in a unique, bell-shaped regulation on blood lipid levels. The novel mechanism ultimately enabled the manganese-based therapy that could clear plaques in mice bearing CVDs.
“We are truly fascinated by manganese’s potential in both preventing and treating the biggest disease,” says Dr. Xiao-Wei Chen, the senior author of the work, “and we are enthusiastic to learn more about its efficacy and safety, as well as developing more efficient ways to harness this novel signaling function of manganese.”
More information:
Yawei Wang et al, Manganese therapy for dyslipidemia and plaque reversal in murine models, Life Metabolism (2023). DOI: 10.1093/lifemeta/load040
Xiao Wang et al, Manganese regulation of COPII condensation controls circulating lipid homeostasis, Nature Cell Biology (2023). DOI: 10.1038/s41556-023-01260-3
Provided by
Higher Education Press
Citation:
A ‘manganese bullet’ targeting cardiovascular disease? Research finds potential therapy for intensive lipid lowering (2023, November 2)
retrieved 2 November 2023
from https://medicalxpress.com/news/2023-11-manganese-bullet-cardiovascular-disease-potential.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.
