Cardiovascular
Weight-loss drug Wegovy could get expanded FDA approval within six months, Novo Nordisk says
- Novo Nordisk said Thursday that its blockbuster weight-loss drug Wegovy could receive expanded approval from the U.S. Food and Drug Administration within six months.
- Chief Financial Officer Karsten Munk Knudsen said the company had received priority review in its application for approval of Wegovy as a treatment for reducing the risk of cardiovascular disease.
- A greenlight from the health agency could potentially boost the insurance coverage prospects of the highly sought-after drug.
Novo Nordisk said Thursday that its blockbuster weight-loss drug Wegovy could receive expanded approval from the U.S. Food and Drug Administration within six months.
Chief Financial Officer Karsten Munk Knudsen told CNBC that the Danish pharmaceutical company had received priority review in its application for approval of Wegovy as a treatment for reducing the risk of cardiovascular disease.
A greenlight from the health agency could potentially boost the insurance coverage prospects of the highly sought-after drug.
“I would say from today, [the outcome will be] less than six months,” Knudsen told CNBC’s Julianna Tatelbaum on “Street Signs.”
Earlier Thursday, Novo Nordisk in its third-quarter earnings announced plans to get expanded approval from the FDA, but shared no timeline. It also reported record profits and sales for the period on the back of the runaway success of its obesity drug.
Growing health applications
Late-stage trial data in August showed that Wegovy reduced the risk of major cardiovascular events such as heart attacks or strokes by 20%, compared with a placebo.
“The SELECT study is, in an obese population with established cardiovascular disease, does Wegovy reduce cardiovascular risk? And the answer is, yes it does, by 20%,” Knudsen said Thursday.
The results of the closely watched “SELECT” trial were seen as a boon for Novo Nordisk’s ambitions of moving beyond Wegovy’s image as a “vanity drug.”
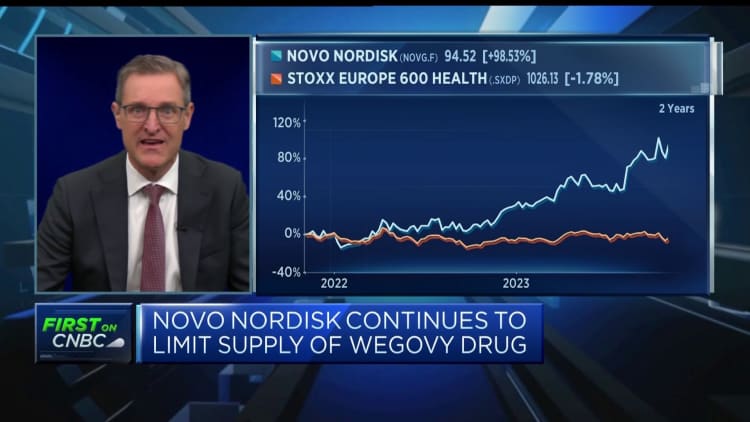
The findings provided an added boost for the company’s stock, which has been on the up this year. Shares were 1.5% higher Thursday morning following its earning’s report; over the year to date, shares are almost 45% higher.
Knudsen said the company will present a detailed copy of the trial findings in approximately 10 days, after which the FDA will have six months to provide its verdict.
He added that approval would expand the use cases of the drug, bolstering the chances of insurance companies paying for the treatment. Some insurers have so far been reluctant to cover the drug — which has a U.S. list price of $1,350 a month — solely for weight-loss purposes.
“The pricing of the products are also a reflection of the value that they give to society,” Knudsen said.
“So the more data we are able to generate, whether it’s on cardiovascular disease or chronic kidney disease or other comorbidities, that is of course part of the overall value story when we discuss with payers and insurers.”
However, even with approval, supply shortages could continue to hamper rollout of the drug. Knudsen said Thursday that the company is continuing to ramp up production.
“I can promise you coming into next year that we’re significantly scaling Wegovy supply,” he said.
