Cancer and neoplasms
Cerebellar Haemangioblastoma with Leptomeningeal spread
Introduction
Hemangioblastomas (HB) are neoplasms originating in the central nervous system (CNS) with a generally good clinical course and prognosis. They represent 1.5–2.5% of all intracranial neoplasms and 7–12% of posterior fossa tumors.1 In about 30% of cases, HB are associated with von Hippel-Lindau syndrome (VHL), an autosomal dominant disorder characterized by lesions in different tissues, although most arise sporadically.2
Various molecular alterations have been described in HB, not only mutations in VHL (substitutions, deletions, insertions, and duplications), but recurrent DNA copy-number losses of chromosomes 6q and 6, and somatic gain-of-function EPAS1 (HIF2A). Various cell signaling mechanisms are upregulated in CNS HB, including EGFR (HER1), TGFα, FGFR3, PDGFRA, and Notch signaling pathways or receptors.3,4
We present a case of sporadic cerebellar HB that recurred eight years after diagnosis, with multifocal leptomeningeal seeding and a fatal outcome, describing the clinical and radiological characteristics as well as the immunohistochemical and molecular alterations in MDM2 and EGFR genes.
Materials and Methods
Detailed methods are freely available in the Data Availability Statement section (see the https link in Data Availability Statement) including the following:
- Clinical and radiological data.
- Histological characteristics
- Immunohistochemistry methods and analysis
- Copy Number Alterations by Fluorescence in Situ Hybridization methods and analysis.
Results
Clinical History and Imaging
A 42-year-old man who complained of suboccipital headaches, which gradually transformed into holocranial headaches. At clinical examination he declared 10 kilogram weight loss, dizziness, unsteady gait, and behavioral disturbances in the last six weeks. He also declared a previous history of human immunodeficiency virus (HIV) infection, diagnosed when he was 35 years old, treated with Efavirenz, Emtricitabine, and Tenofovir disoproxil. Undetected viral load was confirmed in every HIV serology test performed after treatment.
Brain Magnetic Resonance Imaging (MRI) with contrast, revealed an intra-axial mass located in the middle line of the posterior fossa, centered in the cerebellar Vermis, which showed two different well-defined components: one was solid and the other was cystic. The solid component was hypervascular, predominantly located on top. The cystic component, on the lower part of the tumor, showed a T2 high signal intensity and T1 hypointense signal (Figure 1A–C and E). Both tumor components together measured 48x40x35 mm in three dimensions. The mass caused obstructive hydrocephalus with significantly enlarged lateral ventricles (Figure 1B and C), obliteration of the cerebellopontine angle cistern, and tonsillar herniation. The diffusion-weighted magnetic resonance imaging (DW-MRI) as well as the apparent diffusion coefficient (ADC) map, did not show signal restriction on the solid component of the tumor (Figure 1F). Nevertheless, hypervascular areas were exclusively present in the solid component of the tumor. The radiological diagnosis proposed a differential diagnosis including hemangioblastoma (HB), astrocytoma, and metastasis.
|
Figure 1 Brain MRI features: post contrast coronal (B), sagittal (C), and axial (E), T1-weighted sequences. See the obstructive hydrocephalus showing abnormally large lateral ventricles (yellow arrows on (B and C)), and the intra-axial mass located on cerebellar vermis with its solid and cystic components (red arrows on (A, B, C and E)). Cerebellar detail on axial apparent diffusion coefficient (ADC) map (F). Cervical spine MRI features: post contrast, sagittal T2 weighted sequences (A), and sagittal T1 weighted sequences (D). See the cerebellar mass and the multifocal spinal cord with leptomeningeal seeding (red arrows on (A and D)). |
The patient underwent a surgical approach by neuronavigation with cranial nerve monitoring. A suboccipital incision and craniotomy in the left occipital region, near the foramen Magnum, were performed, continuing with a “Y”-shaped durotomy. The tumor was resected from the cerebellar vermis region, bleeding easily during the procedure. A microdissection of the IV ventricle’s floor to achieve a complete tumor removal, was necessary. No early post-surgical complications were reported, nevertheless after a few months, 10th cranial nerve palsy was diagnosed, requiring tracheostomy and percutaneous endoscopic gastrostomy (PEG).
Eight years later, a tumoral recurrence with supra and infratentorial involvement with multifocal spinal cord and leptomeningeal seeding was found on MRI (Figure 1A and D). Secondary hydrocephalus necessitated various surgical interventions.
Ventriculoperitoneal shunt was needed. After that, ventriculitis was diagnosed. Cerebrospinal fluid (CSF) cytology showed lymphocytic pleocytosis, however tumor cells or pathogenic microorganisms were not reported. The patient was hospitalized due to a respiratory infection (septicemia caused by Pseudomonas aeruginosa) and bacteremia caused by Staphylococcus aureus. The respiratory symptoms disappeared with antibiotics, however neurological status worsened. Brain-CT was performed urgently. Hydrocephalus and intracranial hypertension were observed. After the external ventricular drain, the patient died within a few hours.
Pathological Findings
Gross description: the tumor presented well-defined solid and cystic areas. The largest tumor diameter was 48mm with well-circumscribed and smooth margins. Cut surface showed brown color with hemorrhagic areas.
Microscopically, it consisted of a monotonous component of stromal cells with clear to vacuolated cytoplasm, and small round to oval nucleus intermingled with abundant delicate capillary vessels (Figure 2A).
 |
Figure 2 Histopathological, immunohistochemical, and molecular findings: stromal cells with clear to vacuolated cytoplasm and small round to oval nucleus intermingled with abundant delicate capillary vessels (A). Stromal cells show α-inhibin positivity (B). Capillary elements show VEGF positivity (C). MDM2 by IHC shows nuclear positivity in a high proportion of tumor cells (D), in accordance with the signal gains on MDM2 gene detected by FISH (E). EGFR by IHC shows membranous positivity in all tumor cells (F), also in accordance with the signal gains on EGFR gene detected by FISH (G). |
Immunohistochemical Findings
Immunohistochemically, the stromal cell component showed immunoreactivity for Inhibin alpha (Figure 2B), S100, neuron specific enolase (NSE), CD56, MDM2, and EGFR. Interestingly, PAX8 and Carbonic anhydrase IX (CaIX) were also positive. The vascular elements showed positivity for CD31, ERG, GLUT1, and VEGF (Figure 2C). Finally, EMA, CD10, PAX2, and Synaptophysin were negative (Data Availability Statement).
Fluorescence in situ Hybridization Findings
The analysis of MDM2 and EGFR by fluorescent probes (FISH) revealed that 73% of stromal cells showed 5 copies (red and green signals) of MDM2 gene (12q15) (Figure 2E); 52% of stromal tumor cells showed 5 copies (red signals) of EGFR gene (7p11) but polysomies were also observed in 25% of stromal tumor cells (four green signals and two red signals) (Figure 2G). Gains on these genes were also found in four more cases (from the series of 12 HB originally analyzed), (Data Availability Statement).
Discussion
Hemangioblastoma (HB) is a central nervous system (CNS) WHO grade 1, highly vascular tumor, containing neoplastic stromal cells that have clear to vacuolated cytoplasm and characteristic immunohistochemical features (eg, Inhibin alpha positivity) and molecular findings (eg, von Hippel–Lindau (VHL) alterations).3 HB accounts for < 2% of all CNS tumors, and they may occur sporadically or in association with VHL-syndrome,5,6 a familial predisposition to develop different types of cancer, such as pheochromocytoma, paraganglioma, retinal- and cerebellar-hemangioblastoma, and renal cell carcinoma (RCC).7 Our patient did not have a family history of VHL alterations. Neither somatic nor germline mutations were found on VHL molecular analysis.
Cellular subtype with clear cell aggregates may initially be misdiagnosed as metastatic clear cell renal cell carcinoma (ccRCC). Immunoreactivity for Inhibin alpha and D2-40, in addition to negative staining for RCC (antibody), EMA, CD10, and CAM5.2 antibodies, may be helpful for excluding metastatic renal cell carcinoma. PAX8 and CaIX can be positive in both, therefore we recommend not using them in differential diagnosis.8
Overexpression of EGFR in VHL-related CNS HB has been described by Liu et al.9 They found that the expression of EGFR in VHL-related HB was significantly higher than that in the control group by IHC (EGFR, p = 0.007) and by RT-PCR (p = 0.017). Böhling et al, also found that the stromal cells in HB showed high and uniform EGFR expression by IHC.10
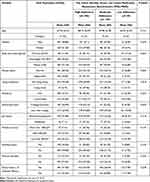
Copy-number alterations (CNAs) in various genes, including gain of EGFR and microdeletion of FGFR1, have been identified in HB.3,11 Relevant literature studies about EGFR alterations in hemangioblastoma are compiled in Table 1. We found copy number gains in EGFR gene by FISH, in good concordance with IHC results (Figure 2F and G), (Data Availability Statement).
 |
Table 1 Relevant Literature Studies about EGFR Alterations in Hemangioblastoma |
There are not many scientific publications about the implications of MDM2 alteration in the oncogenesis and evolution of HB. Falconieri et al, predicted that MDM2 is a novel direct interactor of pVHL.14 To our knowledge, this is the first publication in English literature in which MDM2 gene was analyzed by IHC and FISH in a series of sporadic HB cases (non VHL-related HB) and specifically in a case with a fatal outcome. We found copy-number gains in MDM2 gene by FISH in good concordance with IHC results (Figure 2D and E), (Data Availability Statement).
The prognosis of HB is generally excellent after a complete excision. Early treatment is highly recommended to avoid permanent neurological deficits.15 Sporadic HB has a better prognosis compared to VHL-associated ones, among other reasons, because the latter shows multifocality. Features that impact prognosis include age, size, growth pattern, treatment, multifocality, and location.16
Thus, our case presents some unusual characteristics rarely found in sporadic HB, such as the leptomeningeal spread, local recurrence, and complication with a fatal outcome, being more frequently found in VLH-related forms of HB. There is not enough scientific evidence, to date, that allows us to conclude that the EGFR and MDM2 findings observed in our case induced a clinically worse and fatal outcome. This requires a deeper molecular investigation with a large prospective series to shed light on the role of protein expression on IHC, and CNAs on FISH, in EGFR and MDM2 genes, in the oncogenesis of HB and its potential significance in therapy and prognosis.
Abbreviations
HB, hemangioblastoma; CNS, central nervous system; VHL-gene, von Hippel-Lindau gene; VHL-syndrome, von Hippel-Lindau syndrome; pVHL, von Hippel–Lindau protein; FFPE, formalin-fixed paraffin-embedded; IHC, immunohistochemistry; FISH, fluorescence in situ hybridization; CNAs, copy number alterations; RT-PCR, reverse transcription-polymerase chain reaction; EGFR, epidermal growth factor receptor; MDM2, murine double minute 2; TKIs-EGFR, EGFR tyrosine kinase inhibitors; EGFR1, epidermal growth factor receptor 1; HER1, human epidermal growth factor receptor 1; EPAS1, Endothelial PAS Domain Protein 1; HIF2A, hypoxia-inducible factor 2-alpha; TGF-α, transforming growth factor-alpha; FGFR3, fibroblast Growth Factor Receptor 3; PDGFRA, platelet Derived Growth Factor Receptor Alpha; CD56, cluster of differentiation 56; CD31, cluster of differentiation 31; CaIX, carbonic anhydrase IX; PAX8, paired box gene 8; PAX2, paired box gene 2; VEGF, vascular endothelial growth factor; ERG, erythroblast transformation-specific [ETS]-related gene; GLUT-1, glucose transporter 1; EMA, epithelial membrane antigen; CD10, cluster of differentiation 10; HIV, human immunodeficiency virus; MRI, Magnetic Resonance Imaging; DW-MRI, diffusion-weighted magnetic resonance imaging; ADC, apparent diffusion coefficient; Brain-CT, computed tomography of the brain; PEG, percutaneous endoscopic gastrostomy; WHO, World Health Organization.
Data Sharing Statement
The data are publicly available in the following link:
https://github.com/LMCT-Repository/MDM2-and-EGFR-Alterations-in-HaemangioblastomaHemangioblastoma.git.
Ethics Approval Statement
This manuscript has been reviewed and authorized by the ethics committee of the University Hospital of Salamanca. No private personal data of the patient have been revealed in this manuscript.
Patient and Patient’s Family’s Consent Statement
The patient’s family authorized and consented to the publication. The informed consent signed by the patient’s family included in the clinical data contemplates the scientific disclosure of relevant findings if the privacy of personal data is respected.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
No funding was received by the authors to make this article.
Disclosure
The authors have no conflicts of interest to declare for this work.
References
1. Hussein MR. Central nervous system capillary haemangioblastoma: the pathologist’s viewpoint. Int J Exp Pathol. 2007;88(5):311–324. doi:10.1111/j.1365-2613.2007.00535.x
2. Wanebo JE, Lonser RR, Glenn GM, Oldfield EH. The natural history of hemangioblastomas of the central nervous system in patients with von Hippel-Lindau disease. J Neurosurg. 2003;98(1):82–94. doi:10.3171/jns.2003.98.1.0082
3. Tihan T, Fanburg-Smith JC, Oliver Vortmeyer A, Zagzag D. Haemangioblastoma. In: Central Nervous Tumours Who Classification of Tumours. 5th ed. Lyon, France: International Agency for Research on Cancer (IARC); 2021.
4. Shankar GM, Taylor-Weiner A, Lelic N, et al. Sporadic hemangioblastomas are characterized by cryptic VHL inactivation. acta neuropathol commun. 2014;2(1):167. doi:10.1186/s40478-014-0167-x
5. Nguyen HS, Doan NB, Gelsomino M, et al. Intracranial hemangioblastoma – A SEER-based analysis 2004–2013. Oncotarget. 2018;9(46):28009–28015. doi:10.18632/oncotarget.25534
6. Muscarella LA, la Torre A, Faienza A, et al. Molecular dissection of the VHL gene in solitary capillary hemangioblastoma of the central nervous system. J Neuropathol Exp Neurol. 2014;73(1):50–58. doi:10.1097/NEN.0000000000000024
7. Minervini G, Quaglia F, Tabaro F, Tosatto SCE, Schlessinger A. Genotype-phenotype relations of the von Hippel-Lindau tumor suppressor inferred from a large-scale analysis of disease mutations and interactors. PLoS Comput Biol. 2019;15(4):e1006478. doi:10.1371/journal.pcbi.1006478
8. North PE, Mizeracki A, Mihm MC, Mrak RE. GLUT1 immunoreaction patterns reliably distinguish hemangioblastoma from metastatic renal cell carcinoma. Clin Neuropathol. 2000;19(3):131–137.
9. Liu Z, Li L, Yi Z, et al. Overexpression of EGFR and TGFα in von Hippel-Lindau related central nervous system hemangioblastomas. Front Oncol. 2020;10:703. doi:10.3389/fonc.2020.00703
10. Böhling T, Hatva E, Kujala M, Claesson-Welsh L, Alitalo K, Haltia M. Expression of growth factors and growth factor receptors in capillary hemangioblastoma. J Neuropathol Exp Neurol. 1996;55(5):522–527. doi:10.1097/00005072-199605000-00004
11. Mehrian-Shai R, Yalon M, Moshe I, et al. Identification of genomic aberrations in hemangioblastoma by droplet digital PCR and SNP microarray highlights novel candidate genes and pathways for pathogenesis. BMC Genomics. 2016;17(1):56. doi:10.1186/s12864-016-2370-6
12. Reifenberger G, Reifenberger J, Bilzer T, Wechsler W, Collins VP Coexpression of transforming growth factor-alpha and épidermal growth factor receptor in capillary hemangioblastomas of the central nervous system. Am J Pathol. 1995;147(2):245-250.
13. Chen GJ, Karajannis MA, Newcomb EW, Zagzag D Overexpression and activation of epidermal growth factor receptor in hemangioblastomas. J Neurooncol. 2010;99(2):195-200. doi:10.1007/s11060-010-0125-9
14. Falconieri A, Minervini G, Bortolotto R, et al. The E3 ubiquitin-protein ligase MDM2 is a novel interactor of the von Hippel-Lindau tumor suppressor. Sci Rep. 2020;10(1):15850. doi:10.1038/s41598-020-72683-3
15. Ordookhanian C, Kaloostian PE, Ghostine SS, Spiess PE, Etame AB. Management strategies and outcomes for VHL-related craniospinal hemangioblastomas. J Kidney Cancer VHL. 2017;4(3):37–44. doi:10.15586/jkcvhl.2017.90
16. Huang Y, Chan L, Bai HX, et al. Assessment of care pattern and outcome in hemangioblastoma. Sci Rep. 2018;8(1):11144. doi:10.1038/s41598-018-29047-9