Cancer and neoplasms
Analysis of PD-1 inhibitors in pancreatic cancer
Zhitao Chen,1,* Yahui He,2,* Chenchen Ding,3 Jun Chen,2 Yangjun Gu,1 Min Xiao,1 Qiyong Li1
1Hepatobiliary and Pancreatic Surgery, Shulan (Hangzhou) Hospital Affiliated to Zhejiang Shuren University Shulan International Medical College, Hangzhou, 310003, People’s Republic of China; 2School of Medicine, Zhejiang Chinese Medical University Zhejiang Shuren College, Hangzhou, 310003, People’s Republic of China; 3Child and Adolescent Psychology, Affiliated Mental Health Centre & Hangzhou Seventh People’s Hospital, Zhejiang University School of Medicine, Hangzhou, Zhejiang, 310013, People’s Republic of China
Correspondence: Qiyong Li, Hepatobiliary and Pancreatic Surgery, Shulan (Hangzhou) Hospital Affiliated to Zhejiang Shuren University Shulan International Medical College, 848# Dongxin Road, Hangzhou, Zhejiang Province, 310003, People’s Republic of China, Tel +86-0571-56757021, Email [email protected]
Background: Pancreatic cancer is a deadly disease with a low five years survival rate, and chemotherapy remains the standard treatment for advanced cases. However, the efficacy of chemotherapy alone is limited, and there is a need for new treatment options. Recently, immune checkpoint inhibitors (ICIs), particularly programmed death-1 (PD-1) inhibitors, have shown promising results in various cancers, including pancreatic cancer. In this study, we explore the safety and efficacy of PD-1 inhibitors in combination with chemotherapy for advanced pancreatic cancer.
Materials and Methods: A retrospective analysis was conducted on clinical data from 27 patients with advanced pancreatic cancer who were administered a combination of anti-PD-1 antibody and gemcitabine plus nab-paclitaxel (GnP) regimen. The study evaluated the safety of the treatment as well as the objective response rate (ORR), disease control rate (DCR), progression-free survival (PFS), and overall survival (OS).
Results: In this study, treatment with a combination of anti-PD-1 antibody and GnP regimen for pancreatic cancer resulted in partial response (PR) for 10 out of 27 (37.04%) patients, stable disease (SD) for 10 (37.04%) patients, and progressive disease (PD) for 7 (25.92%) patients. The study found that the median OS (mOS) for these patients was 16.4 months [standard error (SE) = 1.117, 95% confidence interval (CI) 14.211– 18.589], while the median PFS (mPFS) was 6.4 months (SE = 1.217, 95% CI 3.981– 8.752). Subgroup analysis revealed that pancreatic cancer patients’ Eastern Cooperative Oncology Group (ECOG) performance status (PS) (0 vs 1) and treatment cycles (≤ 6 cycles vs > 6 cycles) significantly affected OS and PFS. Patients experienced mostly grade 1– 2 adverse events (AEs), which were relieved through clinical treatment.
Conclusion: The combination of GnP with anti-PD-1 antibodies shows promise as a potential treatment option for advanced pancreatic cancer.
Keywords: pancreatic cancer, immune checkpoint inhibitors, PD-1, gemcitabine, nab-paclitaxel, chemotherapy
Introduction
Pancreatic cancer is one of the first malignant cancers of the digestive system with the highest mortality.1,2 Pancreatic ductal adenocarcinoma (PDAC), the twelfth most common human malignant tumor and the seventh-leading cause of cancer-related deaths worldwide, is the major pathological subtype of pancreatic neoplasms.2,3 Cigarette smoking, alcohol consumption, chronic pancreatitis, family history of pancreatic cancer, diabetes and obesity are the well-recognized risk factors linked to this malignancy.4–8 Surgical resection and lymph node dissection is currently considered to be the most potential treatment to achieve a favorable prognosis, yet only approximately 25% of patients with pancreatic cancer are operable at first diagnosis.9,10 For patients with metastatic, recurrent, or advanced pancreatic cancer, comprehensive treatment including chemotherapy, radiotherapy, targeted therapy, and immunotherapy is extremely important to improve survival.11,12 However, there was no consensus about optimal multimodality treatment for patients with unresectable pancreatic cancer.
Currently, chemotherapy is still the dominant and most effective treatment for patients with metastatic and advanced pancreatic cancer based on National Cancer Comprehensive Network (NCCN) guidelines.13 FOLFIRINOX (5-fluorouracil + Leucovorin + Irinotecan + Oxaliplatin) or gemcitabine-based chemotherapy has been commonly used as first-line chemotherapy in clinical practice.13,14 Numerous clinical trials involving patients with unresectable pancreatic cancer have reported promising results; however, chemotherapy alone did not significantly improve outcome.15 Meanwhile, the clinical application of FOLFIRINOX is restricted due to high incidence of severe toxicity, including grade 3 or 4 neutropenia and febrile neutropenia.16,17 Furthermore, a previous clinical trial demonstrated that gemcitabine plus nab-paclitaxel therapy (GnP) had superior overall survival (OS) and median progression free survival (mPFS) of 8.5 and 5.5 months, respectively, compared to gemcitabine alone for patients with unresectable locally advanced or metastatic pancreatic cancer.15 In recent years, immune-oncology (IO) therapies is a therapeutic treatment option for cancer patients by stimulating patient immune systems to fight cancer. Of all the IO therapies, immune checkpoint inhibitors (ICIs), a monoclonal antibodies (mAbs) that block immunosuppressive pathways, are extensively used in clinical practice as antineoplastic drugs, with multiple Food and Drug Administration (FDA) approvals across solid tumors.18 Programmed death-1 (PD-1) have been studied for years as one of the most promising ICIs and used in the treatment of solid tumors to bring significant benefit.19 While pancreatic cancer is generally regarded as an immunologically cold tumor resistant to immunotherapy, clinical observations have shown that there can be instances of efficacy.20 The role of anti-PD-1 antibody combined with GnP regimen for advanced pancreatic cancer remains debatable due to the conflicting results from different clinical trials and retrospective studies. Based on previous studies, advanced pancreatic cancer is selected as the research object in present study for the exploration of the efficacy, safety, and impact on patient survival of PD-1 blockade combined with GnP regimen.
Despite the ongoing emergence of promising results and the accumulation of clinical data on the combination of GnP therapy with PD-1 inhibitors in the clinical treatment of pancreatic cancer, existing clinical guidelines have refrained from recommending it due to its inconsistent efficacy. Therefore, the present study retrospectively collected clinical data on the GnP regimen in pancreatic cancer combined with immunotherapy, aiming to provide additional clinical evidence for future assessments of the suitability of immunotherapy for pancreatic cancer patients.
Materials and Methods
Patients
For the current retrospective study, a total of 27 unresectable locally advanced or metastatic pancreatic cancer patients with PD-1 plus GnP from Shulan (Hangzhou) hospital between February 20, 2019 and June 21, 2021. Patients with postoperative relapse PDAC were eligible for inclusion in present study. The inclusion criteria were as follows: (i) diagnosed with unresectable locally advanced or metastatic PDAC by pathology according to the NCCN clinical practice guideline.21 (ii) age between 18 and 70 years. (iii) received PD-1 in combination with GnP treatment as the first-line regimen. (iiii) at least 1 measurable lesion in line with Response Evaluation Criteria in Solid Tumors (RECIST, version 1.1). The exclusion criteria were as follows: (i) The patients with other malignant tumors including primary solid tumors or hematologic malignancies. (ii) incomplete patient clinical data. (iii) Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 2 to 4.
Treatment
In our study, all patients received PD-1 in combination with GnP treatment in a 21 days cycle. PD-1 was administered once every 3 weeks. All PD-1 information and dosage are summarized in Table 1. The GnP regimen involved gemcitabine at 1000 mg/m2 and nab-paclitaxel at 125 mg/m2 on days 1, 8 of a 21 days cycle. Treatment continued until disease progression or death, occurrence of uncontrollable adverse events (AEs), self-termination of treatment.
 |
Table 1 The Immune Checkpoint Inhibitors (ICIs) Information and Dosage |
Follow-Up and Outcome Evaluation
Patients were followed up until October 28, 2022 or until death via hospitalization, outpatient, or telephone. The follow-up included routine history, physical examination, routine blood, liver, kidney and thyroid functions, tumor markers and abdominal computed tomography (CT) or magnetic resonance imaging (MRI). AEs were graded using National Cancer Institute Common Terminology Criteria for Adverse Events22 (NCI-CTCAE, version 4.0) (The work was completed by two senior clinicians). Radiographic results were evaluated according to RECIST (version 1.1) every 3 cycles of the treatment (The work was completed by two senior imaging diagnosis physicians). The OS, progression free survival (PFS), disease control rate (DCR), objective response rate (ORR) and AEs were evaluated. The OS period was defined as the time period between the time of surgery and the time of death, or it was censored at the time of the last follow-up. The PFS was defined as the time until clinical-proven tumor progression.
Statistical Analysis
All statistical analyses were performed using R Software (version 4.2.2) and SPSS (version 25). Categorical variables were described using frequencies and percentages, and non-normally distributed data were expressed as medians with interquartile range (IQR). Both OS and PFS were estimated using the Kaplan–Meier method. Meanwhile, Hazard Ratio (HR) and 95% Confidence Interval (CI) were obtained using Cox regression analysis. The ORR was defined as the proportion of the total number of complete responses (CR) and partial responses (PR), whereas DCR was defined as the sum of ORR and stable disease (SD). Time to response (TTR) was calculated from the start of treatment to the achievement of response. The time to disease progression (TTP) was defined as the period between the start of treatment and the onset and advancement of the tumor. The duration of response (DOR) was defined as the time from the first documented response to the date of progression or death.
Results
Clinical Characteristics
A total of 27 patients with locally advanced or metastatic pancreatic cancer received PD-1 in combination with GnP treatment between February 20, 2019 and June 21, 2021 in Shulan (Hangzhou) Hospital. The basic clinical characteristics of these patients are listed in Table 2. The median age of 27 analyzed PDAC patients was 64 years (range from 46 to 77 years) and included 9 (9/27 33.3%%) women and 18 (18/27 66.7%) men. This was associated with the fact that pancreatic cancer is an aged male-dominant cancer. Among all patients, 6 cases (6/27, 22.2%) had the tumor in the head of the pancreas while for 21 (21/27, 77.8%) cases it was in the body and tail of the pancreas. All the patients were well ECOG PS (0 or 1). Sixteen (59.2%) patients could not be operated due to locally advanced stage and 11 patients (40.8%) had liver metastasis. Among these patients, 9 patients received pembrolizumab, 9 patients received sintilimab, 4 patients received camrelizumab, 3 patients received tislelizumab, 1 patient received toripalimab, and 1 patient received nivolumab.
|
Table 2 Clinical Characteristics of 27 Patients with Pancreatic Cancer |
Best Treatment Response
The follow-up was ended in December 28, 2022. After treatment, 10 (37.04%) patients achieved PR, 10 (37.04%) patients got SD and 7 (25.92%) patients achieved PD. No pancreatic cancer patient had a CR from PD-1 in combination with GnP treatment. Of note, one patient had a remarkable tumor shrinkage after receiving 4 times of PD-1 in combination with GnP treatment, and received surgical treatment after multi-disciplinary treatment (MDT) discussion (Figure 1). Furthermore, ORR and DCR were 37.04% and 74.08%, respectively. The TTR, TTP, and DOR were 2.87 (IQR 2.17–3.30), 4.87 (IQR 3.57–8.13), and 4.15 (IQR 2.61–6.02), respectively (Table 3). In this swimmer plot, each bar indicates the duration of response status of each patient (Figure 2).
|
Table 3 The Treatment Response of 27 Pancreatic Cancers Received PD-1 in Combination with GnP Treatment |
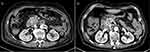 |
Figure 1 Enhanced computed tomography images of the abdomen. (A) Before treatment, there was a malignant tumor located in the head of the pancreas. (B) A malignant tumor has been detected in the pancreatic head of a patient who underwent gemcitabine plus nab-paclitaxel (GnP) chemotherapy along with immunotherapy. |
|
Figure 2 Swimmer plot that displays the response status of pancreatic cancer patients treated with gemcitabine plus nab-paclitaxel (GnP) chemotherapy in conjunction with immunotherapy. |
Survival
Until the final follow-up time, 27 patients (100%) experienced disease progression after received PD-1 in combination with GnP treatment and 22 patients (81.5%) had died. The median treatment cycle was 7.0 cycles (range, 3–14 cycles). Among these pancreatic cancer patients, the median OS was 16.4 months [standard error (SE) = 1.117, 95% confidence interval (CI) 14.211–18.589] (Figure 3A), and the median PFS was 6.4 months (SE = 1.217, 95% CI 3.981–8.752) (Figure 3B). The 1-year OS rates were 66.7%, and 2-year OS rates were 18.5%, whereas the 6-months PFS rates were 51.9% and 12-months PFS rates were 0.
 |
Figure 3 The Kaplan-Meier survival curves of patients with pancreatic cancer who were treated with a combination of gemcitabine plus nab-paclitaxel (GnP) chemotherapy and immunotherapy were evaluated. (A) The overall survival (OS) of all patients with pancreatic cancer who were treated with a combination of gemcitabine plus nab-paclitaxel (GnP) chemotherapy and immunotherapy was evaluated. (B) The progression-free survival (PFS) of all patients with pancreatic cancer who were treated with a combination of gemcitabine plus nab-paclitaxel (GnP) chemotherapy and immunotherapy was evaluated. |
Predictors of Survival
In terms of the relationship between patients’ different characteristics with OS, we found that hepatic metastasis [yes vs no, p < 0.001, Hazard Ratio (HR) = 5.643, 95% CI 1.577–20.193, Figure 4A], treatment cycles (6 cycles, p = 0.008, HR = 0.262, 95% CI 0.092–0.751, Figure 4B) and ECOG PS (0 vs 1, p = 0.003, HR = 8.470, 95% CI 2.513–28.544, Figure 4C) were significantly associated with the OS of pancreatic cancer. However, sex (male vs female, p = 0.776, HR = 0.869, 95% CI 0.329–2.295, Figure 4D), age (62y, p = 0.163, HR = 0.480, 95% CI 0.168–1.373, Figure 4E), Carbohydrate antigen 19–9 (CA19-9) level (high vs low, p = 0.518, HR = 1.619, 95% CI 0.371–7.072, Figure 4F), carcinoembryonic antigen (CEA) level (high vs low, p = 0.851, HR = 0.920, 95% CI 0.386–2.194, Figure 4G), and tumor location (body and tail of pancreas vs pancreatic head, p = 0.950, HR = 0.959, 95% CI 0.265–3.471, Figure 4H) were not related to OS. In terms of PFS, ECOG PS (0 vs 1, p = 0.029, HR = 3.159, 95% CI 1.061–9.411, Figure 5A) and treatment cycles (6 cycles, p < 0.001, HR = 0.003, 95% CI 0.000005–1.464, Figure 5B) were correlated with PFS of pancreatic cancers, while sex (male vs female, p = 0.498, HR = 0.717, 95% CI 0.272–1.889, Figure 5C), age (62y, p = 0.667, HR = 0.826, 95% CI 0.345–1.978, Figure 5D), CA19-9 level (high vs low, p = 0.681, HR = 1.361, 95% CI 0.310–5.966, Figure 5E), CEA level (high vs low, p = 0.808, HR = 0.896, 95% CI 0.369–2.175, Figure 5F), tumor location (body and tail of pancreas vs pancreatic head, p = 0.223, HR = 2.111, 95% CI 0.616–7.241, Figure 5G), and hepatic metastasis (yes vs no, p = 0.331, HR = 1.615, 95% CI 0.607–4.294, Figure 5H) were not associated with PFS.
|
Figure 4 Kaplan-Meier survival curves were plotted to analyze the overall survival (OS) of patients with pancreatic cancer who received a combination of gemcitabine plus nab-paclitaxel (GnP) chemotherapy and immunotherapy, stratified by different clinical subgroup. (A) OS according to the hepatic metastasis (yes vs no, p < 0.001). (B) OS according to the treatment cycles (6 cycles, p = 0.008). (C) OS according to the Eastern Cooperative Oncology Group (ECOG) performance status (PS) (0 vs 1, p = 0.003). (D) OS according to the sex (male vs female, p = 0.776). (E) OS according to the age (62y, p = 0.163). (F) OS according to the carbohydrate antigen 19–9 (CA19-9) level (high vs low, p = 0.518). (G) OS according to the carcinoembryonic antigen (CEA) level (high vs low, p = 0.851). (H) OS according to the tumor location (body and tail of pancreas vs pancreatic head, p = 0.950). |
|
Figure 5 Kaplan-Meier survival curves were plotted to analyze the progression-free survival (PFS) of patients with pancreatic cancer who received a combination of gemcitabine plus nab-paclitaxel (GnP) chemotherapy and immunotherapy, stratified by different clinical subgroup. (A) PFS according to the Eastern Cooperative Oncology Group (ECOG) performance status (PS) (0 vs 1, p = 0.029). (B) PFS according to the treatment cycles (6 cycles, p < 0.001). (C) PFS according to the sex (male vs female, p = 0.498). (D) PFS according to the age (62y, p = 0.667). (E) PFS according to the carbohydrate antigen 19–9 (CA19-9) level (high vs low, p = 0.681). (F) PFS according to the carcinoembryonic antigen (CEA) level (high vs low, p = 0.808). (G) PFS according to the tumor location (body and tail of pancreas vs pancreatic head, p = 0.223). (H) PFS according to the hepatic metastasis (yes vs no, p = 0.331). |
CA19-9 Level
The tumor marker CA19-9 showed a statistically significant decrease trend after PD-1 in combination with GnP treatment in patients with pancreatic cancer (p < 0.01, Figure 6). However, the reduced CA19-9 level did not exert an effect on OS (p = 0.858, HR = 0.911, 95% CI 0.330–2.517, Figure 7A) and PFS (p = 0.250, HR = 0.550, 95% CI 0.195–1.552, Figure 7B).
|
Figure 6 Variation of carbohydrate antigen 19–9 (CA19-9) before and after treatment in pancreatic cancer patients; two asterisks (**) indicates statistical significance at p < 0.01. |
 |
Figure 7 The relationship between the decline of carbohydrate antigen 19–9 (CA19-9) and the prognosis of pancreatic cancer who received a combination of gemcitabine plus nab-paclitaxel (GnP) chemotherapy and immunotherapy, (A) overall survival (OS); (B)progression-free survival (PFS). |
AEs
The main AEs observed during treatment with PD-1 blockade combined with the GnP regimen in 27 patients with pancreatic cancer are presented in Table 4. The most common AEs were leukopenia (88.9%), neutropenia (88.9%), thrombocytopenia (59.3%), weakness (63.0%), and nausea or vomiting (100%).
 |
Table 4 Adverse Reactions During Treatment of the 27 Patients |
Discussion
Pancreatic cancer is considered one of the most aggressive and deadly forms of cancer due to its tendency to spread rapidly to other parts of the body, the lack of noticeable symptoms in its early stages, and its resistance to traditional chemotherapy.23–25 The survival rate for pancreatic cancer is also among the lowest for all types of cancer, with only about 7% of patients surviving for 5 years or more after diagnosis.26 Nowadays, pancreatic cancer has been recognized as a systematic disease and a multimodality management is highly recommended in NCCN and the Chinese Society of Clinical Oncology (CSCO) practice guideline.12,27 GnP regimen or FOLFIRINOX are widely used as the first-line treatment for advanced pancreatic cancer, and proved to significantly improve survival outcome.13,27 In light of the significant adverse effects associated with the FOLFIRINOX chemotherapy regimen, which can be challenging for patients to tolerate, we opted to utilize the GnP regimen in this study. PD-1 is an immune checkpoint molecule expressed on the surface of T cells, B cells, and Natural killer (NK) cells. It inhibits the immune response of T cells by binding with its ligands PD-L1 and PD-L2.28,29 Immunotherapy targeting the PD-1 signaling pathway has become a hot spot in the field of cancer treatment. Anti-PD-1 monoclonal antibodies (such as nivolumab, pembrolizumab) have been developed as important drugs for cancer immunotherapy. Pancreatic cancer is considered an immunologically cold tumor that is resistant to immunotherapy. It remains controversial whether additional adjuvant immunotherapy would bring a survival benefit.
Pancreatic cancer is known to have a poor response to immunotherapy. There are several reasons why pancreatic cancer has a limited response to immunotherapy, including the immunologically “cold” nature of the tumors, low tumor mutational burden, suppressive immune microenvironment, and tumor heterogeneity.30–32 These challenges make it difficult for the immune system to recognize and attack pancreatic cancer cells, reducing the effectiveness of immunotherapy. In early clinical trials, patients with pancreatic cancer who received PD-1 inhibitors alone did not show a significant improvement in PFS and OS compared to those who did not receive the treatment.33,34 Chemotherapy can enhance the efficacy of immunotherapy for pancreatic cancer by weakening their immune evasion mechanisms, releasing tumor antigens and reducing the number of tumor cells, making it easier for the immune system to identify and attack cancer cells. In the phase Ib/II study (NCT02331251), pembrolizumab combined with GnP resulted in a 27% ORR (3 out of 11 patients with PR) in treatment-naïve metastatic pancreatic cancer patients and 0% ORR in previously treated patients.35 Some recent retrospective study with either single center cohort indicated patients with pancreatic cancer who received PD-1 inhibitors and chemotherapy showed a significant improvement in OS and PFS compared to those who did not receive the treatment.36,37 Del Re et al38 conducted a comparative study to assess the impact of two standardized chemotherapy regimens, FOLFIRINOX (a combination of fluorouracil, irinotecan, and oxaliplatin) and GnP, on the levels of PD-L1 mRNA expression in plasma-derived microvesicles (MVs) of pancreatic cancer patients. They observed that, in comparison to the FOLFIRINOX group, patients in the GnP group exhibited a significant increase in PD-L1 mRNA expression levels in MVs both at baseline and after three months of treatment. These findings provide a theoretical basis for the potential of the GnP regimen to enhance the efficacy of PD-1 inhibitors in pancreatic cancer therapy.38 After observing the positive safety and efficacy results of previous clinical studies involving immune combined chemotherapy, we have also noted similar benefits in patients with advanced pancreatic cancer at our center when using PD-1 combined chemotherapy. Thus, we conducted the present retrospective study to the efficacy, safety, and impact on patient survival of PD-1 blockade combined with GnP regimen.
This study mainly retrospectively collected the advanced pancreatic cancer patients treated with PD-1 blockade combined with GnP regimen for follow-up observation, and explored the curative effect and the incidence of AEs. The result disclosed that CR, PR, SD, and PD rates were 0.0%, 37.04%, 37.04%, and 25.92% in advanced pancreatic cancer patients treated with PD-1 plus GnP regimen chemotherapy, correspondingly. Furthermore, ORR and DCR were 37.04% and 74.08%, respectively. The TTR, TTP, and DOR were 2.87 (IQR 2.17–3.30), 4.87 (IQR 3.57–8.13), and 4.15 (IQR 2.61–6.02). The findings reflected a certain efficacy of PD-1 plus GnP regimen chemotherapy in treating unresectable or advanced pancreatic cancer patients. The reasons may be as follows: First of all, the chemotherapy works by killing rapidly dividing cells, including cancer cells. As cancer cells die, they release antigens that can trigger an immune response.39 In addition, chemotherapy can help to counteract this immunosuppressive environment by reducing the number of regulatory T cells and myeloid-derived suppressor cells, which can prevent cancer cells from evading the immune system.40,41 Furthermore, chemotherapy can also increase the expression of PD-L1, the ligand that interacts with the PD-1 receptor, making cancer cells more vulnerable to PD-1 immunotherapy.41 After establishing the anti-tumor immune response, patients who received ICIs in addition to GnP chemotherapy demonstrated longer long-term survival compared to those who only received chemotherapy.42
The subgroup analysis, interestingly, showed that ECOG PS (0 vs 1) were significantly associated with the OS and PFS of pancreatic cancer. Similarly, patients who underwent six or more treatment cycles, as opposed to fewer than six, had longer OS and PFS. This result can be attributed to the fact that patients who have higher levels of ECOG PS may have a decreased ability to tolerate treatment, leading to a poorer prognosis. Nonetheless, it is possible that patients who undergo a sustained treatment regimen could potentially benefit more from this regimen. We have also discovered that patients with presence of liver metastasis tend to have a worse OS, but this does not seem to have any correlation with PFS. However, a meta-analysis conducted by Usón, which combined data from two Phase III studies, found no significant impact on OS based on the metastatic burden.15,43,44
The common toxic reactions observed during the treatment were bone marrow suppression (leukopenia, neutropenia, thrombocytopenia, and anemia), weakness and nausea. Most patients had grade 1–2 adverse reactions, and the symptoms were relieved after clinical treatment. Very few patients were evaluated as having grade 3–4 immune adverse reactions. Intravenous hormone therapy and immunotherapy after symptom recovery did not affect the treatment cycle, indicating that the side effects are safe and effective in the controllable range.
There are several limitations to our study, primarily related to its retrospective design. Firstly, the study was conducted in a single institution, which may introduce bias regarding treatment choices made by the attending oncologist. Secondly, the interpretation of the subgroup analysis is limited by the relatively small sample size. Additionally, the heterogeneity of therapies applied in later treatment lines may have influenced the OS outcome.
Conclusion
The current study suggests that combining anti-PD-1 antibody with chemotherapy may offer some benefits in the treatment of advanced pancreatic cancer. However, as this was a small-scale retrospective clinical study, it has inherent limitations. Therefore, further research is necessary to determine the optimal use of PD-1 inhibitors in treating pancreatic cancer and to identify patients who are most likely to benefit from this treatment.
Abbreviations
ICIs immune checkpoint inhibitors, PD-1 programmed death-1, GnP gemcitabine plus nab-paclitaxel, ORR objective response rate, DCR disease control rate, PFS progression-free survival, OS overall survival, PR partial response, SD stable disease, PD progressive disease, ECOG Eastern Cooperative Oncology Group, PS performance status, PDAC Pancreatic ductal adenocarcinoma, NCCN National Cancer Comprehensive Network, IO immune-oncology, mAbs monoclonal antibodies, FDA Food and Drug Administration, RECIST Response Evaluation Criteria in Solid Tumors.
Data Sharing Statement
The data that support the findings of this study are available from corresponding author but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of corresponding author.
Ethics Approval and Consent to Participate
This study conforms to the principles outlined in the Declaration of Helsinki (World Medical Association Declaration of Helsinki), all the cases used in this study were approved by the Academic Committee of Shulan (Hangzhou) Hospital (KY2023010). Written informed consent was obtained from the study participants prior to the study commencement.
Acknowledgments
All authors express our gratitude to all the doctors in the Department of Hepatobiliary and Pancreatic Surgery, Department of Oncology, and Department of Radiology at Shulan (Hangzhou) Hospital, Zhejiang Shuren University, for their support.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Park W, Chawla A, O’Reilly EM. Pancreatic Cancer: a Review. JAMA. 2021;326(9):851–862. doi:10.1001/jama.2021.13027
2. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33. doi:10.3322/caac.21654
3. Wong MCS, Jiang JY, Liang M, Fang Y, Yeung MS, Sung JJY. Global temporal patterns of pancreatic cancer and association with socioeconomic development. Sci Rep. 2017;7(1):3165. doi:10.1038/s41598-017-02997-2
4. Bosetti C, Lucenteforte E, Silverman DT, et al. Cigarette smoking and pancreatic cancer: an analysis from the International Pancreatic Cancer Case-Control Consortium (Panc4). Ann Oncol. 2012;23(7):1880–1888. doi:10.1093/annonc/mdr541
5. Jiao L, Berrington de Gonzalez A, Hartge P, et al. Body mass index, effect modifiers, and risk of pancreatic cancer: a pooled study of seven prospective cohorts. Cancer Causes Control. 2010;21(8):1305–1314. doi:10.1007/s10552-010-9558-x
6. Rahn S, Zimmermann V, Viol F, et al. Diabetes as risk factor for pancreatic cancer: hyperglycemia promotes epithelial-mesenchymal-transition and stem cell properties in pancreatic ductal epithelial cells. Cancer Lett. 2018;415:129–150. doi:10.1016/j.canlet.2017.12.004
7. Lucenteforte E, La Vecchia C, Silverman D, et al. Alcohol consumption and pancreatic cancer: a pooled analysis in the International Pancreatic Cancer Case-Control Consortium (PanC4). Ann Oncol. 2012;23(2):374–382. doi:10.1093/annonc/mdr120
8. Gandhi S, de la Fuente J, Murad MH, Majumder S. Chronic Pancreatitis Is a Risk Factor for Pancreatic Cancer, and Incidence Increases With Duration of Disease: a Systematic Review and Meta-analysis. Clin Transl Gastroenterol. 2022;13(3):e00463. doi:10.14309/ctg.0000000000000463
9. Matsuki R, Okano N, Hasui N, et al. Trends in the surgical treatment for pancreatic cancer in the last 30 years. Biosci Trends. 2022;16(3):198–206. doi:10.5582/bst.2022.01250
10. Speer AG, Thursfield VJ, Torn-Broers Y, Jefford M. Pancreatic cancer: surgical management and outcomes after 6 years of follow-up. Med J Aust. 2012;196(8):511–515. doi:10.5694/mja11.10890
11. Mizrahi JD, Surana R, Valle JW, Shroff RT. Pancreatic cancer. Lancet. 2020;395(10242):2008–2020. doi:10.1016/s0140-6736(20)30974-0
12. Tempero MA. NCCN Guidelines Updates: pancreatic Cancer. J Natl Compr Canc Netw. 2019;17(5.5):603–605. doi:10.6004/jnccn.2019.5007
13. Tempero MA, Malafa MP, Al-Hawary M, et al. Pancreatic Adenocarcinoma, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2021;19(4):439–457. doi:10.6004/jnccn.2021.0017
14. Conroy T, Hammel P, Hebbar M, et al. FOLFIRINOX or Gemcitabine as Adjuvant Therapy for Pancreatic Cancer. N Engl J Med. 2018;379(25):2395–2406. doi:10.1056/NEJMoa1809775
15. Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369(18):1691–1703. doi:10.1056/NEJMoa1304369
16. Okusaka T, Ikeda M, Fukutomi A, et al. Phase II study of FOLFIRINOX for chemotherapy-naïve Japanese patients with metastatic pancreatic cancer. Cancer Sci. 2014;105(10):1321–1326. doi:10.1111/cas.12501
17. van Roessel S, van Veldhuisen E, Klompmaker S, et al. Evaluation of Adjuvant Chemotherapy in Patients With Resected Pancreatic Cancer After Neoadjuvant FOLFIRINOX Treatment. JAMA Oncol. 2020;6(11):1733–1740. doi:10.1001/jamaoncol.2020.3537
18. Mukherji R, Debnath D, Hartley ML, Noel MS. The Role of Immunotherapy in Pancreatic Cancer. Curr Oncol. 2022;29(10):6864–6892. doi:10.3390/curroncol29100541
19. Pedoeem A, Azoulay-Alfaguter I, Strazza M, Silverman GJ, Mor A. Programmed death-1 pathway in cancer and autoimmunity. Clin Immunol. 2014;153(1):145–152. doi:10.1016/j.clim.2014.04.010
20. Chick RC, Gunderson AJ, Rahman S, Cloyd JM. Neoadjuvant Immunotherapy for Localized Pancreatic Cancer: challenges and Early Results. Cancers. 2023;15(15):56.
21. Tempero MA, Malafa MP, Al-Hawary M, et al. Pancreatic Adenocarcinoma, Version 2.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2017;15(8):1028–1061. doi:10.6004/jnccn.2017.0131
22. Dueck AC, Mendoza TR, Mitchell SA, et al. Validity and Reliability of the US National Cancer Institute’s Patient-Reported Outcomes Version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE). JAMA Oncol. 2015;1(8):1051–1059. doi:10.1001/jamaoncol.2015.2639
23. Zeng S, Pöttler M, Lan B, Grützmann R, Pilarsky C, Yang H. Chemoresistance in Pancreatic Cancer. Int J Mol Sci. 2019;20(18). doi:10.3390/ijms20184504
24. Wood LD, Canto MI, Jaffee EM, Simeone DM. Pancreatic Cancer: pathogenesis, Screening, Diagnosis, and Treatment. Gastroenterology. 2022;163(2):386–402.e1. doi:10.1053/j.gastro.2022.03.056
25. De Dosso S, Siebenhüner AR, Winder T, et al. Treatment landscape of metastatic pancreatic cancer. Cancer Treat Rev. 2021;96:102180. doi:10.1016/j.ctrv.2021.102180
26. Li S, Xu HX, Wu CT, et al. Angiogenesis in pancreatic cancer: current research status and clinical implications. Angiogenesis. 2019;22(1):15–36. doi:10.1007/s10456-018-9645-2
27. National Health Commission Of The People’s Republic Of C. Chinese guidelines for diagnosis and treatment of pancreatic cancer 2018 (English version). Chin J Cancer Res. 2019;31(2):278–294. doi:10.21147/j.issn.1000-9604.2019.02.03
28. Ravindranathan S, Passang T, Li JM, et al. Targeting vasoactive intestinal peptide-mediated signaling enhances response to immune checkpoint therapy in pancreatic ductal adenocarcinoma. Nat Commun. 2022;13(1):6418. doi:10.1038/s41467-022-34242-4
29. Blair AB, Wang J, Davelaar J, et al. Dual Stromal Targeting Sensitizes Pancreatic Adenocarcinoma for Anti-Programmed Cell Death Protein 1 Therapy. Gastroenterology. 2022;163(5):1267–1280.e7. doi:10.1053/j.gastro.2022.06.027
30. Falcomatà C, Bärthel S, Schneider G, Rad R, Schmidt-Supprian M, Saur D. Context-Specific Determinants of the Immunosuppressive Tumor Microenvironment in Pancreatic Cancer. Cancer Discov. 2023;13(2):278–297. doi:10.1158/2159-8290.Cd-22-0876
31. Karamitopoulou E, Andreou A, Wenning AS, Gloor B, Perren A. High tumor mutational burden (TMB) identifies a microsatellite stable pancreatic cancer subset with prolonged survival and strong anti-tumor immunity. Eur J Cancer. 2022;169:64–73. doi:10.1016/j.ejca.2022.03.033
32. Lawlor RT, Mattiolo P, Mafficini A, et al. Tumor Mutational Burden as a Potential Biomarker for Immunotherapy in Pancreatic Cancer: systematic Review and Still-Open Questions. Cancers. 2021;13(13):68.
33. Patnaik A, Kang SP, Rasco D, et al. Phase I Study of Pembrolizumab (MK-3475; Anti-PD-1 Monoclonal Antibody) in Patients with Advanced Solid Tumors. Clin Cancer Res. 2015;21(19):4286–4293. doi:10.1158/1078-0432.Ccr-14-2607
34. Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455–2465. doi:10.1056/NEJMoa1200694
35. Weiss GJ, Blaydorn L, Beck J, et al. Phase Ib/II study of gemcitabine, nab-paclitaxel, and pembrolizumab in metastatic pancreatic adenocarcinoma. Invest New Drugs. 2018;36(1):96–102. doi:10.1007/s10637-017-0525-1
36. Song D, Yang X, Guo X, Sun H. Safety and efficacy analysis of PD-1 inhibitors in combination with chemotherapy for advanced pancreatic cancer. Immunotherapy. 2022;14(16):1307–1313. doi:10.2217/imt-2022-0196
37. Zhou L, Wu Z, Jiang C, Efficacy DS. Safety, and Impact on Patient Survival of PDL1/PD-1 Inhibitors versus FOLFIRINOX Regimens for Advanced Pancreatic Cancer. Comput Math Methods Med. 2022;2022:5430720. doi:10.1155/2022/5430720
38. Del Re M, Vivaldi C, Rofi E, et al. Gemcitabine Plus Nab-Paclitaxel Induces PD-L1 mRNA Expression in Plasma-Derived Microvesicles in Pancreatic Cancer. Cancers. 2021;13(15):76.
39. Sriram G, Milling LE, Chen JK, et al. The injury response to DNA damage in live tumor cells promotes antitumor immunity. Sci Signal. 2021;14(705):eabc4764. doi:10.1126/scisignal.abc4764
40. Galluzzi L, Humeau J, Buqué A, Zitvogel L, Kroemer G. Immunostimulation with chemotherapy in the era of immune checkpoint inhibitors. Nat Rev Clin Oncol. 2020;17(12):725–741. doi:10.1038/s41571-020-0413-z
41. Gao Z, Bai Y, Lin A, et al. Gamma delta T-cell-based immune checkpoint therapy: attractive candidate for antitumor treatment. Mol Cancer. 2023;22(1):31. doi:10.1186/s12943-023-01722-0
42. Zhang F, Wang Y, Yang F, Zhang Y, Jiang M, Zhang X. The Efficacy and Safety of PD-1 Inhibitors Combined with Nab-Paclitaxel Plus Gemcitabine versus Nab-Paclitaxel Plus Gemcitabine in the First-Line Treatment of Advanced Pancreatic Cancer: a Retrospective Monocentric Study. Cancer Manag Res. 2022;14:535–546. doi:10.2147/cmar.S349442
43. Usón PLSJ, Tolentino FDS, Santos VM, Rother ET, Maluf FC. The impact of metastatic sites in advanced pancreatic adenocarcinoma, systematic review and meta-analysis of prospective randomized studies. PLoS One. 2020;15(3):e0230060. doi:10.1371/journal.pone.0230060
44. Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817–1825. doi:10.1056/NEJMoa1011923

