Cancer and neoplasms
Enduring questions in regenerative biology and the search for answers
Abstract
The potential for basic research to uncover the inner workings of regenerative processes and produce meaningful medical therapies has inspired scientists, clinicians, and patients for hundreds of years. Decades of studies using a handful of highly regenerative model organisms have significantly advanced our knowledge of key cell types and molecular pathways involved in regeneration. However, many questions remain about how regenerative processes unfold in regeneration-competent species, how they are curtailed in non-regenerative organisms, and how they might be induced (or restored) in humans. Recent technological advances in genomics, molecular biology, computer science, bioengineering, and stem cell research hold promise to collectively provide new experimental evidence for how different organisms accomplish the process of regeneration. In theory, this new evidence should inform the design of new clinical approaches for regenerative medicine. A deeper understanding of how tissues and organs regenerate will also undoubtedly impact many adjacent scientific fields. To best apply and adapt these new technologies in ways that break long-standing barriers and answer critical questions about regeneration, we must combine the deep knowledge of developmental and evolutionary biologists with the hard-earned expertise of scientists in mechanistic and technical fields. To this end, this perspective is based on conversations from a workshop we organized at the Banbury Center, during which a diverse cross-section of the regeneration research community and experts in various technologies discussed enduring questions in regenerative biology. Here, we share the questions this group identified as significant and unanswered, i.e., known unknowns. We also describe the obstacles limiting our progress in answering these questions and how expanding the number and diversity of organisms used in regeneration research is essential for deepening our understanding of regenerative capacity. Finally, we propose that investigating these problems collaboratively across a diverse network of researchers has the potential to advance our field and produce unexpected insights into important questions in related areas of biology and medicine.
Introduction
Modern scientific research generally reflects Thomas Kuhn’s notion of normal science, whereby technological advances enable researchers to pursue incremental confirmations of existing theory1. This approach does, on occasion, produce unexpected insight into outstanding problems, but its fidelity to conventional frameworks discourages the sort of creative experimentation and custom tool-building that produces paradigm-shifting results. Moreover, deploying new technology just because it is available can create the illusion of advancement, i.e., posing questions that have already been answered with less-advanced methods but are proffered as unresolved in order to apply the latest sophisticated (and usually expensive) technology. This is often done with good intentions, e.g., the hope of discovering something new with more sensitive detection or detail, but ultimately does not actually remove the most critical barriers that must be surmounted to move a field forward2. Furthermore, this kind of “modern science” incentivizes specialization and, in doing so, focuses effort away from bigger questions that require interdisciplinary efforts and have the potential to advance multiple fields.
In an attempt to think beyond “normal science,” we wrote this perspective piece to synthesize and share ideas discussed at a Banbury Center workshop on enduring questions in regenerative biology (Box 1). As a group broadly representative of the regeneration field, we reflected on the significant progress made in the past four decades and discussed the types of ambitious community-led projects that we need to pursue to uncover answers to new and enduring questions. There was a strong feeling that by doing this collectively and with the input of experts from other disciplines, regeneration researchers would be better prepared to harness existing or new technologies and design experiments that can begin to address these questions. When scientific collectives assemble to examine major problems, it motivates collaborative efforts across research groups and disciplines. Knowledge creation in one field often spurs progress in related areas, generating benefits for science far beyond the original goals.
Despite the varied expertise among workshop participants and wide-ranging discussions about how regeneration occurs among diverse species, we found broad agreement on identifying several enduring, fundamental questions where scientists should direct their efforts. Importantly, our task was to identify common problems that are not overly reductionist or specific to a particular organism. In essence, we focused on the forest to identify major driving questions while at the same time considering why some trees remain undescribed, hidden, or unknown. We found common ground on the notion that regeneration remains vastly understudied, in part because the most commonly used model organisms do not have robust regenerative capacities. Unsurprisingly, there was no strong impetus for creating new technologies to specifically study regeneration; the enduring technological hurdle in our field is the application of specific transgenic tools to highly regenerative research models. Thus, the perspective put forth here represents a synthesis of focused discussions that aim to stimulate an exchange of ideas and future collaborations between experts in many fields, including regeneration.
Which key processes comprise regeneration?
Despite general agreement for defining regeneration (see Box 2), what remains ill-defined is the set of component processes that comprise regeneration from induction to resolution. While it is clear that regeneration is induced by significant tissue loss or wounding, when and how regeneration-specific processes can be distinguished from those that occur during wound healing and fibrotic repair remain unresolved. Historically, many researchers studied tissue repair mechanisms holistically across diverse species despite varied healing outcomes, i.e., fibrotic or regenerative3. However, modern (1980s-present) wound/tissue repair research has largely been siloed from epimorphic regeneration research because studies on wound repair rely heavily on mice, rats, and humans (i.e., non-regenerative species in which wound healing normally generates scar tissue). Similarly, research into epimorphic regeneration has relied on a few highly regenerative invertebrate and vertebrate models (e.g., Hydra, planarians, zebrafish, salamanders). As regeneration research expands its scope to include new research organisms that can be genetically manipulated and maintained in a laboratory setting4 or that enable comparisons of regenerative success and failure between closely related and divergent species, our definition of “regenerative capacity” needs re-evaluation. For instance, does a species’ “regenerative capacity” signify the inheritance of a single, albeit complex, trait or a combination of separate, interwoven processes? If the latter, are all component processes required for successful regeneration, or might some tissues/species omit one or more? Conversely, do all non-regenerative organisms diverge at the same stage of this progression? Ultimately, refining the definition of regenerative capacity returns us to a fundamental question: what are the component events that comprise regeneration? Defining these components allows one to determine the extent to which they share similarities with or are distinct from processes that occur during fibrotic repair. Thus, we identified a set of fundamental processes that occur across most regenerative species (summarized in Table 1).
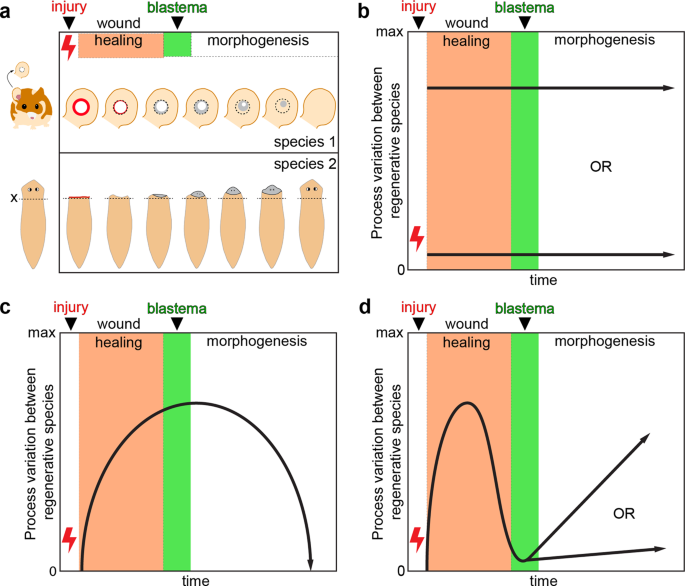
After defining component processes common to regeneration, we considered how these events might vary across an ever-broadening set of organisms. For instance, complex tissue regeneration may be evolutionarily constrained such that the entire regenerative response, including all component processes, is canalized with low variation between species for any specific process or set of interrelated processes (Fig. 1a, b). This could be true even if regenerative ability has evolved multiple times in different lineages. Conversely, regeneration could have arisen via convergent evolution (i.e., homoplasy) and variability among component processes may be large across the entire process set or for most of the processes (Fig. 1c). Alternatively, variation across species could be relatively large for some processes yet low for others, i.e., high conservation of specific processes (Fig. 1d). Defining the processes outlined in Table 1 as component parts of regeneration provides a framework to study each one across organisms and facilitates the generation of testable hypotheses to ask what is lacking (or modified) in non-regenerative organisms. For example, one testable hypothesis is that events one through five outlined in Table 1 constitute a general wound response that occurs in all animals, regardless of subsequent steps, with regeneration requiring a distinct mechanism that transitions tissues into regenerative healing (steps six through nine, Table 1). Another hypothesis is that specific events occur during the early wounding response to trigger regenerative healing.
a Two example species that exhibit epimorphic regeneration: Acomys cahirinus (spiny mouse) and Schmidtea mediterranea (freshwater planarian). Complex tissue regeneration of the ear pinna (spiny mouse) and head (planarian) is depicted as occurring from an initial tissue injury through complete regeneration. Individual processes as presented in Table 1 are contained within three general phases of regeneration: wound healing, blastema formation, and morphogenesis. Because the timescale over which these processes occur in individual species is highly variable, regeneration in a is depicted independent of time. b–d Three alternative hypotheses describing variability between comparable processes across regenerative species as a function of time. b Variability between component processes during the time course of regeneration is either low (i.e., genetic, molecular, and cellular mechanisms are highly conserved across species, bottom arrow) or high (mechanisms are not well conserved, upper arrow). c The initial injury response between species is very similar, but variability increases for mechanisms associated with cell activation, cell cycle progression, blastema formation, and morphogenesis, and then becomes more similar again during the differentiation and scaling phases of regeneration. The hypothesis represented in d asserts that variation between species is relatively large for processes that occur during wound healing, but low in the processes involved in blastema formation; after blastema formation, process variability may remain low (conserved) or increase (divergent).
In line with the second hypothesis, one enduring question is whether a particular injury signal predicts the final healing outcome. For example, is there a unique trigger for wound healing versus regeneration5? Many investigators have advanced the hypothesis that particular immune cells and their products are specifically required for regeneration, independent of their role in regulating and resolving inflammation6. For instance, studies in several adult regeneration models suggest blocking immune cell infiltration (e.g., monocytes and macrophages) or depleting specific immune cell subtypes (e.g., macrophages) prevents normal wound healing and the transition to regenerative healing7,8,9,10,11. In contrast, removing similar immune cell types when trying to stimulate regeneration can enhance the response (microglia)12. However, it remains an open question if immune phenotypes exist that regulate and promote regeneration-specific processes. The hypothesis that regeneration-promoting immune cell states exist could be tested by comparing immune system responses across different types of injuries in the same species, i.e., where one injury induces regeneration and the other does not (e.g., lizard tails vs. limbs). This hypothesis could also be tested by comparing the immune response in different aged animals or closely related species in which identical tissues heal via regeneration and fibrotic repair, respectively. Comparing the regenerative response in divergent regenerative species may also offer insight into whether components of the immune response are permissive or instructive relative to regeneration.
Component processes and the transitions between them are also critical to define because they establish a framework for generating specific datasets (i.e., cell type and time-point specific) that can capture cell state changes to compare across species. Specifically, changes in chromatin state or genomic architecture, which impact gene regulatory networks (GRNs) and their outputs, may be broadly conserved at these cellular and temporal transitions13. Importantly, cell state changes accompanying reactivation of developmental genes can provide signposts for exploring chromatin states associated with activation or repression of gene expression and thus provide insight into both activation and constraint of regenerative ability14. These types of comparisons also lay the groundwork for evaluating cell-type evolution models as they relate to the presence or absence of regenerative capacity15,16. Lastly, breaking regeneration into component processes provides a framework for comparing cellular transitions as they occur during regeneration and embryonic development (Boxes 3 and 4).
What constitutes the beginning of regeneration?
Cells detect and respond to injury regardless of the healing outcome (regeneration or scarring), which raises another outstanding question: to what degree do different healing trajectories overlap (Fig. 2a–d)? For example, the ERK/MAPK signaling pathway is rapidly induced upon tissue injury in both non-regenerating and regenerating species and inhibition of its activation impairs wound healing and regeneration5,17,18,19,20,21. What remains unclear is whether the requirement of ERK/MAPK signaling in regeneration directly results from its role in wound healing or if this pleiotropic pathway induces multiple downstream mechanisms that are separately required for multiple regeneration steps (Table 1). Similarly, are there inductive molecules with the dual capacity to promote regeneration and antagonize fibrosis?22 In broader terms, is it possible to identify a set of conserved cellular and molecular mechanisms that initiate regeneration and thus define the “beginning” of regeneration? As discussed above, it remains unclear whether the early events of wound healing are common across most species and contexts with the unique regenerative response initiated later, or if the different healing trajectories (regeneration vs. scarring) are established during the healing process (Fig. 2c, d). If the latter, how can we discover molecular signals and cell states specific to regeneration?

a, b Examples of variation in regenerative ability across species. a Tissue healing is quite different between closely related (~18 mya) Acomys cahirinus (spiny mouse) and Mus musculus (laboratory mouse; Adobe Stock Image used with educational license) where Acomys exhibit complex tissue regeneration in the ear pinna and identical injuries heal with scar tissue (and no regeneration) in Mus. b While two flatworm species Schmidtea mediterranea (orange) and Dendrocoelum lacteum (gray) are capable of head regeneration, D. lacteum exhibits poor head regeneration from posterior fragments (S. mediterranea has near limitless regenerative ability from any fragment). c Comparing regeneration and fibrotic repair, the early events that occur prior to new tissue formation could be similar between species, only to diverge as mechanisms specific to regeneration or fibrotic repair are activated. In the example presented, this divergence occurs immediately prior to activation of a developmental state (i.e., blastema formation), although under this hypothesis it could occur later. d An alternative is that regenerative and fibrotic healing are evolutionarily distinct and thus upon injury two different healing trajectories, and their mechanistic underpinnings, are expressed.
To distinguish between regenerative responses and more general repair processes, it can be useful to identify events that occur during both regeneration and embryonic development. As embryogenesis unfolds in a temporal sequence beginning from fertilization, tissues, and organs arise at precise positions and prescribed times based on a specific developmental plan. Although it is also difficult to ascribe an initiating event in organ development, developmental biologists largely agree that the formation of a tissue anlagen or primordium is associated with precursor cells that are competent to receive inductive signals. In response to inductive signals (cell-autonomous or non-cell-autonomous), precursor cells launch a developmental program and self-organize into tissues comprising a diverse array of differentiated cell types. Thus, it may be appropriate to consider the beginning of regeneration per se as the point when injury-activated cells accumulate at the injury site and adopt a development-like state (Table 1). This would align the beginning of regeneration with anlagen formation and distinguish wound healing from events specific to regeneration (Fig. 2c).
In support of this proposition, data from salamanders suggests that injury-induced cell accumulation is necessary but insufficient for regeneration (reviewed in ref. 23). Across countless experiments, researchers performed surgical interventions to study how nerve-secreted signals regulate the regenerative response in ambystomid salamanders and newt limbs24,25. These results show that wound healing and proliferative cell accumulation occur after denervating a limb, but aggregated cells fail to progress to morphogenesis. In further support of this idea, inhibiting certain signaling pathways (e.g., Wnt and Hedgehog signaling) allows damaged tissue to repair, but regeneration does not occur26,27,28. Together, these findings support the concept that the ability to accumulate proliferative cells is a necessary but insufficient feature that distinguishes wound healing processes from regeneration. Instead, it suggests the transition to regeneration is characterized by proliferative cells acquiring the ability to undergo patterning, differentiation, and growth, a state often referred to as blastema formation29. However, data linking signaling pathways and nerves to regenerative competence also points to the existence of other essential cellular and molecular events that are required for regeneration to begin in a robust and recognizable way26,27,28. Thus, while the blastema is a common element of what may represent an evolutionarily conserved regenerative feature, it also highlights the critical need to determine which specific cellular and molecular characteristics of the local cellular environment are essential for regeneration to proceed.
The discussion of molecular signals that initiate regeneration evokes broader questions about what initiates the start of regeneration. For example, how is the amount of loss or damage requiring regeneration detected? Are specific regenerative programs only initiated after significant cell loss? If so, what are the mechanisms that activate them? There are many unknowns regarding injury-sensing mechanisms. Are there particular molecules or physiological changes communicating the amount or extent of tissue damage? Does the loss of cell-cell contacts act additively or in parallel to chemical sensing mechanisms? Does the new interaction of cells from different anatomical positions stimulate the regenerative program30,31,32? Overall, the common theme that emerges from these questions is the need to expand data collection on regenerative ability within and across species, particularly by adding data from more examples of failed or intermediate regenerative success. More comprehensive data will allow researchers to better identify cellular, molecular, and functional commonalities in successful regenerative processes versus unsuccessful ones.
What constitutes the end of a regenerative process?
Indisputably, the most desirable outcome of a regeneration process is to regain tissue shape and function. Thus, understanding and defining when and how a regenerative process restores a functional state is equally important to determining how it starts. Most descriptions of tissue or whole-body regeneration indicate that repair processes shift to a remodeling and growth/scaling phase after the initial expansion of cellular material and patterning33. But exactly how regeneration restores form and function is not clearly defined. For example, as cells accumulate and proliferate, how do they appropriately regulate pathways that terminate cell cycle progression and how is this balanced with tissue scaling? Work on the Hippo pathway has demonstrated that its signals can be modulated to regulate growth during development34. A similar use of this pathway likely controls growth during regeneration34,35. However, precisely clarifying when regenerative healing and tissue restoration shift to a growth phase and identifying the molecular mechanisms that regulate this transition is a major objective for our field.
One possibility is that the end of regeneration recapitulates the patterning and growth of embryonic organs, where growth ultimately becomes regulated at the organismal level. For example, regeneration in molting animals (e.g., crustaceans) produces a miniature facsimile of the original appendage, which is only capable of additional growth upon subsequent molts36,37,38. In zebrafish, caudal fin regeneration is generally consistent with the overall size of the animal, but genetic mutants do exist in which regenerating fins lose their allometry and produce dramatically overgrown fins39. Nonetheless, regeneration generally restores missing tissues, their structures, and their functions, suggesting that although the precise mechanisms underlying the integration of new and existing tissue may vary across species, they achieve a common output.
In trying to understand why regeneration is absent in some animal species or tissue types, one hypothesis is that regeneration initiators are missing or not sufficiently activated, while another suggests that some animals lack the capacity to create restorative cell states. Another hypothesis is that mechanisms that normally terminate phases of regenerative healing have been co-opted to inhibit regeneration completely. For example, the evolution of “molecular brakes” in some animals may have rendered certain tissues regeneration-incompetent (e.g., heart muscle and the auditory epithelium)40. The acquisition of molecular breaks is also observed within an animal’s lifetime: neonatal mammals can regenerate a subset of tissues, including cardiac muscle41. Notably, cardiac muscle has been observed to regenerate in newborn mice for approximately one week before this ability is lost42. Could this apparent loss of regenerative ability as the animal ages result from inhibitory mechanisms that halt a specific component process? Comparisons across regenerative versus non-regenerative species/tissues are likely to assist in exploring this hypothesis29. In addition, a greater emphasis on pinpointing mechanisms that terminate or inhibit regenerative processes in cases where regeneration appears to be interrupted or halted after successful initiation may have profound implications for understanding how reduced regenerative capacity evolved in certain animal lineages.
Notably, unbridled overgrowth of regenerating tissue is rarely observed, supporting the hypothesis that regenerative species have mechanisms that provide tight control over proliferation and morphogenesis. Furthermore, reports of tumors in highly regenerative species are uncommon in the literature, although not unknown (reviewed in43,44). Neoplasms can be induced in newts45 and zebrafish (reviewed in46), and epidermal neoplasms occur with some frequency in captive salamander colonies (see “De-Mystifying Salamander Cancers” Facebook group). In spite of these observations, the idea that highly regenerative organisms live tumor-free and are resistant to developing cancer remains a widely held belief43,47. Interestingly, epimorphic regeneration often requires highly conserved pathways that are associated with cancer, and disrupting tumor suppressor genes such as Hippo, p53, or PTEN in planarians and zebrafish can occasionally lead to the formation of tumor-like structures48,49,50,51. Thus, while the incidence of significant, bona fide tumorigenesis in highly regenerative animals appears low, it seems likely that pathways commonly dysregulated in human cancer are tightly controlled during regenerative healing52,53,54,55. Alternatively, the expansion of tumor suppressor gene families in mammals may correlate with their reduced ability to regenerate54. For example, the human ARF protein produced as an alternately spliced gene product from the p16 locus appears to be absent from the genomes of many regenerative species54. In addition, injecting newt myotubes with a plasmid encoding the human tumor suppressor p16INK4a blocks de-differentiation and cell cycle re-entry56. Therefore, tumor suppressor genes may represent another layer of complexity that negatively regulates regenerative responses in mammals. Unraveling the functional relationship between tumor suppression, cell cycle control, and regeneration will require either in vivo genome editing or the introduction of genetically edited cells. However, these tools are currently limited to only a few regenerative organisms.
How does patterning happen across different scales to generate proportioned organs and tissues?
Understanding the mechanisms by which organisms set and restore size and shape is a formidable challenge in development and regeneration. Evidence from divergent organisms indicates that patterning and growth during regeneration depend on the activation of major components of developmental GRNs and signaling pathways. For example, a conserved role for Wnt signaling in re-establishing polarity during regeneration is supported by work in many regeneration systems, including Hydra, acoels, planarians, and amphibians13,27,57,58. Despite redeploying conserved developmental pathways, these processes need to operate on very different timescales and, in some cases, across orders of magnitude in scale, which remains difficult to reconcile with current knowledge about patterning in fields of cells.
In one illustration of how organisms can cope with scaling problems, Drosophila embryos balance cell proliferation and apoptosis to regulate proportion during development. Specifically, Bicoid protein is distributed in a gradient across the developing embryos and organizes anterior development59,60. Yet classic experiments revealed that increases in Bicoid dosage levels, which expand the anterior fate map of the embryo and should give rise to patterning defects, can produce normal larvae60. This restoration of proper proportion is driven by increased cell death in the expanded anterior regions, suggesting that the embryos have mechanisms for sensing and adjusting the relative proportion of anterior fated cells61. Cell death has also been implicated as a key process required in Hydra, planarian, and mammalian liver regeneration62. But how do cells detect when there are too few or too many cells and adjust the rate of proliferation or cell death? How do they maintain the correct ratio of specific cell types, particularly in a replacement tissue that must match the scale of the existing animal? Clearly, these are complex, challenging problems that are relevant to both development and regeneration, making experiments in one context informative for the other.
Besides balancing cell proliferation and death to achieve a proper number of building blocks, injured tissues must specify the fates of new cells and rearrange them appropriately to establish tissue size, proportion, and function. One historical view of how this is accomplished is that cells carry information in a Cartesian code to interpret positional details, and this code is deployed in response to morphogen gradients for pattern formation63. A molecular version of this theory is that cells have different chromatin and transcriptomic states based on their developmental history and can both emit and respond to signals in their local environment. Therefore, overall patterning can vary depending on the type or strength of the signals present and the state of cells interpreting the morphogenic cues. A striking example of the importance of this type of injury-induced communication is the dysregulation of canonical Wnt signaling in planarians, which disrupts anteroposterior polarity and leads to the regeneration of ectopic heads or tails57. In addition, the Wnt-signaling pathway also plays a role in regulating the proportion of new tissues, as disrupting the striatin-interacting phosphatase and kinase (STRIPAK) complex increases worm length by expanding the posterior wnt1 signaling center and dysregulating axial scaling64.
Important regeneration and growth signals are likely not restricted to secreted ligands and well-known signaling pathways. They likely involve ECM components, biomechanical inputs, redox state fluctuations, and changes in metabolic states65,66,67,68. Ion sensing has also been linked to organ size and regeneration69. For example, the zebrafish mutant longfin exhibits fin overgrowth due to ectopic expression of the ion channel Kcnh2a39. Thus, ion sensing and regulation may be a genetically encoded and tunable mechanism for “reading” positional information and producing the correct amount of growth. However, we do not yet know what controls the strength of different types of signals in different contexts and how the regulatory pathways in development might differ from those activated during regeneration. Additionally, although particular signaling pathways may be conserved across contexts, the exact cellular and molecular mechanisms in which they are deployed may differ depending on the size, proportion, and types of tissue being replaced. To this end, embryos typically develop on a much smaller scale than the replacement tissue that is produced during restorative regeneration, so while embryonic pathways may be re-used during regeneration, there are likely key mechanistic differences in how they are employed during the latter.
Is regeneration driven by gene regulatory modules that are conserved across species?
One rapidly expanding area of interest in regeneration biology is the application of genomic tools to understand how gene expression is regulated over time, in different injury and tissue contexts, and between species. Many recent studies have focused on identifying regulatory elements, particularly enhancers, that are potentially unique or specifically activated after injury in regenerative organisms/tissues (see Box 5). These efforts are rational extensions of studies showing that enhancers are sites of dynamic genome interactions with gene promoters during cell fate transitions in various developmental contexts and organisms70,71,72,73,74. Further, the genetic tools and cell culture systems used routinely in Drosophila and ex vivo mammalian cells to dissect regulatory mechanisms are not yet optimized in most highly regenerative organisms. Thus, it is reasonable to use paradigms emerging from traditional models as the basis for targeted experiments in regenerative organisms as a starting point for uncovering gene regulatory modules that function during regeneration. However, given that the genome regulatory mechanisms that drive regenerative processes and transitions may be different from those operating in non-regenerative species, it is also critical for our field to expand our hypotheses and approaches beyond those that emerge from studies in common animal models. To ensure that we identify the key regulatory mechanisms, including potentially novel ones, that control essential regenerative processes, we must invest the resources needed to customize problem-specific tools in regenerative models. For example, there are chemical biology tools (e.g., degrons, azide-labeled non-canonical amino acids) that are not commonly used in our fields but would allow us to address specific, mechanistic questions arising from decades of observational and functional studies.
Any individual group studying genome regulation during regeneration will likely find that evaluating all possible mechanisms of genome regulation is a formidable challenge. Thus, innovation and impactful discovery in this area will particularly benefit from coordinated interaction and collaboration across research groups so that experimental design and genomic data collection can be readily comparable. In addition, a broad view of genomic regulation across multiple regenerative species will allow us to identify conserved mechanisms, even if the specific genetic modules they activate or silence are different13 (Fig. 3). Whether there is greater conservation of upstream genome regulatory mechanisms, the specific proteins orchestrating them, or the genetic modules they target is not likely to have a single or simple answer (Fig. 3). However, we can better distinguish which molecular mechanisms are functionally significant during regeneration by focusing on how specific genetic modules that are known to be activated and essential for regeneration are regulated in regenerative versus non-regenerative contexts.

a In this model, specific gene modules (e.g., coding genes and enhancers) are conserved among various species but regulated differently after injury. This model represents situations where specific genes (e.g., gene X) are present in multiple species, but only activated after injury in regenerative species. It also reflects the possibility that injury-induced genes are activated by different mechanisms in different regenerative species. b In this model, injury induces similar genome regulatory mechanisms (e.g., changes in histone modifications and the expression of pioneering transcription factors) but they have different functional outcomes due to evolutionary differences in the presence and arrangement of specific genetic modules (e.g., the enhancer is only present at this locus in regenerative organisms).
For example, in the regeneration-competent zebrafish retina, expression of transcription factor ascl1 is induced upon retinal injury and required for retinal regeneration75,76. Interestingly, Ascl1 is conserved in mice and required for their retinal development77,78, but Ascl1 expression is not induced after injury of the non-regenerative mouse retina79,80. This observation inspired experiments in which Asl1 was ectopically expressed in explanted adult mouse retinas81. However, although Ascl1 functions like a pioneer factor in some ways (e.g., inducing transcription of some relevant target genes), ectopic Ascl1 expression alone was insufficient to induce functional de-differentiation, proliferation, or redifferentiation in injured mouse retinas81. Yet, importantly, further experiments found that treatment of mouse retinas with ectopic Ascl1 expression in combination with a histone deacetylase (HDAC) inhibitor (which broadly increases genome accessibility) is significantly more successful in activating productive de-differentiation, proliferation, and redifferentiation of mouse cells82. These results are exciting, as they suggest it is indeed possible to reactivate existing genes and induce regeneration in non-regenerative tissues83. Nevertheless, many unanswered questions remain: are HDACs facilitating Ascl1 binding to the genome, and if so, at which loci? Which of these newly bound Ascl1 loci are functionally important, and for which steps in the regenerative processes? Why is the induced regeneration of mouse retinas still less robust than the endogenous regeneration process in zebrafish? What regulatory mechanisms or targeted gene expression programs are missing (or inhibitory)? Experiments addressing these specific questions have the potential to both further our understanding of retinal regeneration and uncover widely conserved molecular mechanisms that regulate essential genetic modules in other regenerative contexts.
From a broader perspective, there are many interesting and unanswered questions regarding the role of genome structure and function in regenerative processes. For example, is it relevant that many regenerative organisms, including vertebrates (e.g., axolotls) and invertebrates (e.g., planarians), have large genomes containing significant amounts of repetitive sequence? What do the repetitive sequences signify? Are these sequences serving as regulatory platforms for transcription factor binding, or do they play more instructive roles in regulating cellular plasticity? Interestingly, studies in mammalian stem cells and early embryos suggest that transient activation of repetitive regions plays a major role in regulating gene expression and chromatin state at critical developmental transitions84,85,86. Are similar mechanisms operating during cell state transitions after an injury? As with the other major knowledge gaps in our field, more comparative studies across species and developmental contexts will be needed to unravel the answers to these questions.
Concluding remarks
One of the oldest and most enduring questions identified by regenerative and developmental biologists is why regenerative ability is unevenly distributed among metazoans87. Why, with over two centuries of regeneration research behind us and major technological advances in molecular biology, next-generation sequencing, and genetic engineering occurring at an ever-quickening pace, does this problem continue to challenge our field? First, evolutionary problems that span large phylogenetic distances are notoriously difficult to address experimentally, and debates about the adaptive nature of regenerative ability remain unresolved. Second, tackling this question necessitates expanding the diversity of species used in regeneration studies88, which faces significant financial and practical barriers. Understandably, many scientists prefer to take advantage of the extensive toolkits available in a small subset of genetic model organisms and shy away from the risks associated with learning or developing a new model system. Of course, the decision to study a new model or species should be driven by scientific questions.
In our discussions at the Banbury workshop, there was broad consensus that comparative studies are essential to identifying the possible cell states, mechanisms, and functions critical for those processes outlined in Table 1. Unfortunately, experimental workflows designed for one species are rarely practical across multiple species due to various confounding factors (e.g., regeneration timing, anatomical differences, genome quality disparities, the need for species-specific expertise, etc.). Instead, the field should invest in collectively designing experimental pipelines that can be deployed across species in individual laboratories with high fidelity. Such pipelines would use existing technologies to generate data that may not be significant in any species but could synergize to generate and address many testable hypotheses. Multi-species studies can provide a platform to test the widely held position that a core regenerative program is conserved among metazoans, and its unequal distribution reflects loss and re-emergence in distant lineages. As the number of species used in regeneration research grows within taxonomic groups and across increasingly distant lineages, it will also provide an opportunity to rigorously examine if the alternative hypothesis may be true: that regeneration has independently evolved in numerous lineages. If the latter hypothesis was shown to be true, it would radically expand potential avenues to explore in regenerative therapies.
Adding to the complexity of discovering the mechanistic basis for interspecific differences in regenerative ability, cellular changes associated with aging (e.g., mutations, metabolism, epigenetic states) are gaining recognition as complementary problems with solutions that may help unlock the potential to stimulate regeneration in humans. Suggestively, animals with the capacity for whole-body regeneration, like Hydra and planarians, appear to be negligibly senescent89, animals with indeterminate growth have high regenerative ability90, and recent work in spiny mice suggests connective tissue cells from these regenerative mammals are highly resistant to stress-induced cellular senescence91. Moreover, long-lived animals and those with post-metamorphic life stages often lose or have diminished regenerative capacities in adulthood compared to their fetal, neonatal, or juvenile life stages92. While the cellular and molecular mechanisms underlying this loss remain poorly understood, possibilities include changes in how cells sense injury signals, cell-autonomous features that prevent cell activation or cell state alternations, or a decline in homeostatic cellular and tissue renewal (Table 1 and Box 2). Together, it is increasingly clear that major advances in dissecting the molecular logic of regeneration (and reconstructing it in humans) cannot occur by studying a handful of organisms. Instead, expanding the collective effort of many research labs across an increasing diversity of organisms can provide answers to some enduring questions in animal biology while potentially unlocking new breakthroughs in human regenerative medicine. Excellent examples of such efforts include comparative studies contrasting species of salamanders, fish, or flatworms8,93,94,95,96, which revealed cellular and molecular insights.
Of course, science at the scale we suggest requires coordination across organisms, labs, and institutions, not to mention a financial strategy to support such activities. Additionally, there is a need for investigators to invest in multiple approaches to rigorously test conclusions on which subsequent studies are based. Rigor and reproducibility, once a cornerstone of the scientific method, have taken a distant backseat to novelty97. Potentially insightful work demonstrated in one model system, or species is strengthened, not weakened, through repeated experimental testing by multiple groups and through testing in other regenerative organisms98. We must ask this of our field. Cross-species study reproduction and hypothesis testing provide another dimension related to the conservation and canalization of regenerative mechanisms. To understand the fundamental principles of regeneration, we urge our peers and other scientists to revisit some of the most enduring questions in our field.
References
-
Kuhn, T. S. The structure of scientific revolutions. 2nd edn. (Chicago University Press, 1970).
-
Kaelin, W. G. Jr Publish houses of brick, not mansions of straw. Nature 545, 387 (2017).
Google Scholar
-
Needham, A. E. Regeneration and wound-healing. (J. Wiley, 1952).
-
Goldstein, B. & Srivastava, M. Emerging model systems in developmental biology. First edition. (Elsevier/Academic Press, 2022).
-
Owlarn, S. et al. Generic wound signals initiate regeneration in missing-tissue contexts. Nat. Commun. 8, 2282 (2017).
Google Scholar
-
Godwin, J. W. & Brockes, J. P. Regeneration, tissue injury and the immune response. J. Anat. 209, 423–432 (2006).
Google Scholar
-
Godwin, J. W., Pinto, A. R. & Rosenthal, N. A. Macrophages are required for adult salamander limb regeneration. Proc. Natl. Acad. Sci. USA 110, 9415–9420 (2013).
Google Scholar
-
Lai, S. L. et al. Reciprocal analyses in zebrafish and medaka reveal that harnessing the immune response promotes cardiac regeneration. Elife 6, e25605 (2017).
-
Petrie, T. A., Strand, N. S., Yang, C. T., Rabinowitz, J. S. & Moon, R. T. Macrophages modulate adult zebrafish tail fin regeneration. Development 141, 2581–2591 (2014).
Google Scholar
-
Simkin, J., Gawriluk, T. R., Gensel, J. C. & Seifert, A. W. Macrophages are necessary for epimorphic regeneration in African spiny mice. Elife 6, e24623 (2017).
-
Vonk, A. C. et al. Single-cell analysis of lizard blastema fibroblasts reveals phagocyte-dependent activation of Hedgehog-responsive chondrogenesis. Nat. Commun. 14, 4489 (2023).
Google Scholar
-
Todd, L., Finkbeiner, C., Wong, C. K., Hooper, M. J. & Reh, T. A. Microglia suppress Ascl1-induced retinal regeneration in mice. Cell Rep. 33, 108507 (2020).
Google Scholar
-
Srivastava, M. Beyond casual resemblance: rigorous frameworks for comparing regeneration across species. Annu. Rev. Cell Dev. Biol. 37, 415–440 (2021).
Google Scholar
-
Lin, T. Y. et al. Fibroblast dedifferentiation as a determinant of successful regeneration. Dev. Cell 56, 1541–1551.e1546 (2021).
Google Scholar
-
Arendt, D. et al. The origin and evolution of cell types. Nat. Rev. Genet. 17, 744–757 (2016).
Google Scholar
-
Tarashansky, A. J. et al. Mapping single-cell atlases throughout Metazoa unravels cell type evolution. Elife 10, e66747(2021).
-
Dieckgraefe, B. K., Weems, D. M., Santoro, S. A. & Alpers, D. H. ERK and p38 MAP kinase pathways are mediators of intestinal epithelial wound-induced signal transduction. Biochem. Biophys. Res. Commun. 233, 389–394 (1997).
Google Scholar
-
Manuel, G. C., Reynoso, R., Gee, L., Salgado, L. M. & Bode, H. R. PI3K and ERK 1-2 regulate early stages during head regeneration in hydra. Dev. Growth Differ. 48, 129–138 (2006).
Google Scholar
-
Tasaki, J. et al. ERK signaling controls blastema cell differentiation during planarian regeneration. Development 138, 2417–2427 (2011).
Google Scholar
-
Fan, Y. et al. Ultrafast distant wound response is essential for whole-body regeneration. Cell 186, 3606–3618.e3616 (2023).
Google Scholar
-
Tomasso, A., Koopmans, T., Lijnzaad, P., Bartscherer, K. & Seifert, A. W. An ERK-dependent molecular switch antagonizes fibrosis and promotes regeneration in spiny mice (Acomys). Sci. Adv. 9, eadf2331 (2023).
Google Scholar
-
Allanki, S. et al. Interleukin-11 signaling promotes cellular reprogramming and limits fibrotic scarring during tissue regeneration. Sci. Adv. 7, eabg6497 (2021).
Google Scholar
-
Stocum, D. L. The role of peripheral nerves in urodele limb regeneration. Eur. J. Neurosci. 34, 908–916 (2011).
Google Scholar
-
Farkas, J. E. & Monaghan, J. R. A brief history of the study of nerve dependent regeneration. Neurogenesis (Austin) 4, e1302216 (2017).
Google Scholar
-
Kumar, A. & Brockes, J. P. Nerve dependence in tissue, organ, and appendage regeneration. Trends Neurosci. 35, 691–699 (2012).
Google Scholar
-
Guimond, J. C. et al. BMP-2 functions independently of SHH signaling and triggers cell condensation and apoptosis in regenerating axolotl limbs. BMC Dev. Biol. 10, 15 (2010).
Google Scholar
-
Kawakami, Y. et al. Wnt/beta-catenin signaling regulates vertebrate limb regeneration. Genes Dev. 20, 3232–3237 (2006).
Google Scholar
-
Schnapp, E., Kragl, M., Rubin, L. & Tanaka, E. M. Hedgehog signaling controls dorsoventral patterning, blastema cell proliferation and cartilage induction during axolotl tail regeneration. Development 132, 3243–3253 (2005).
Google Scholar
-
Seifert, A. W. & Muneoka, K. The blastema and epimorphic regeneration in mammals. Dev. Biol. 433, 190–199 (2018).
Google Scholar
-
Lheureux, E. Nouvelles donnees sur les roles de la peau et des tissues internes dans la regeneration du membre du triton Pleurodeles waltlii, Michah (amphibian urodele). Wilhelm. Roux. Arch. 776, 285–301 (1975).
Google Scholar
-
Lheureux, E. Régénération des membres irradiés dePleurodeles waltlii Michah.(Urodčle). Influence des qualités et orientations des greffons non irradies. Wilhelm Roux’Archiv für Entwicklungsmechanik der Organismen 176, 303–327 (1975).
Google Scholar
-
Carlson, B. M. Morphogenetic interactions between rotated skin cuffs and underlying stump tissues in regenerating axolotl forelimbs. Dev. Biol. 39, 263–285 (1974).
Google Scholar
-
Sanchez Alvarado, A. & Tsonis, P. A. Bridging the regeneration gap: genetic insights from diverse animal models. Nat. Rev. Genet. 7, 873–884 (2006).
Google Scholar
-
Moya, I. M. & Halder, G. Hippo-YAP/TAZ signalling in organ regeneration and regenerative medicine. Nat. Rev. Mol. Cell Biol. 20, 211–226 (2019).
Google Scholar
-
Lin, A. Y. T. & Pearson, B. J. Yorkie is required to restrict the injury responses in planarians. PLoS Genet. 13, e1006874 (2017).
Google Scholar
-
Morgan, T. H. Regeneration. (The Macmillan Company, 1901).
-
Almazan, A., Cevrim, C., Musser, J. M., Averof, M. & Paris, M. Crustacean leg regeneration restores complex microanatomy and cell diversity. Sci. Adv. 8, eabn9823 (2022).
Google Scholar
-
Cooper, R. L. Development of sensory processes during limb regeneration in adult crayfish. J. Exp. Biol. 201, 1745–1752 (1998).
Google Scholar
-
Stewart, S. et al. longfin causes cis-ectopic expression of the kcnh2a ether-a-go-go K+ channel to autonomously prolong fin outgrowth. Development 148. https://doi.org/10.1242/dev.199384 (2021).
-
Cigliola, V., Ghila, L., Chera, S. & Herrera, P. L. Tissue repair brakes: a common paradigm in the biology of regeneration. Stem Cells 38, 330–339 (2020).
Google Scholar
-
Porrello, E. R. et al. Transient regenerative potential of the neonatal mouse heart. Science 331, 1078–1080 (2011).
Google Scholar
-
Montero, A. M. & Huang, A. H. The regenerative capacity of neonatal tissues. Development 149. https://doi.org/10.1242/dev.199819 (2022).
-
Arenas Gomez, C. M. & Echeverri, K. Salamanders: The molecular basis of tissue regeneration and its relevance to human disease. Curr. Top. Dev. Biol. 145, 235–275 (2021).
Google Scholar
-
Amatruda, J. F. & Patton, E. E. Genetic models of cancer in zebrafish. Int. Rev. Cell Mol. Biol. 271, 1–34 (2008).
Google Scholar
-
Zilakos, N. P., Tsonis, P. A., Del Rio-Tsonis, K. & Parchment, R. E. Newt squamous carcinoma proves phylogenetic conservation of tumors as caricatures of tissue renewal. Cancer Res. 52, 4858–4865 (1992).
Google Scholar
-
Stern, H. M. & Zon, L. I. Cancer genetics and drug discovery in the zebrafish. Nat. Rev. Cancer 3, 533–539 (2003).
Google Scholar
-
Robert, J. Comparative study of tumorigenesis and tumor immunity in invertebrates and nonmammalian vertebrates. Dev. Comp. Immunol. 34, 915–925 (2010).
Google Scholar
-
de Sousa, N., Rodriguez-Esteban, G., Rojo-Laguna, J. I., Salo, E. & Adell, T. Hippo signaling controls cell cycle and restricts cell plasticity in planarians. PLoS Biol. 16, e2002399 (2018).
Google Scholar
-
Oviedo, N. J., Pearson, B. J., Levin, M. & Sanchez Alvarado, A. Planarian PTEN homologs regulate stem cells and regeneration through TOR signaling. Dis. Model. Mech. 1, 131–143 (2008). discussion 141.
Google Scholar
-
Faucherre, A., Taylor, G. S., Overvoorde, J., Dixon, J. E. & Hertog, J. Zebrafish pten genes have overlapping and non-redundant functions in tumorigenesis and embryonic development. Oncogene 27, 1079–1086 (2008).
Google Scholar
-
Pearson, B. J. & Sanchez Alvarado, A. A planarian p53 homolog regulates proliferation and self-renewal in adult stem cell lineages. Development 137, 213–221 (2010).
Google Scholar
-
Pardal, R., Molofsky, A. V., He, S. & Morrison, S. J. Stem cell self-renewal and cancer cell proliferation are regulated by common networks that balance the activation of proto-oncogenes and tumor suppressors. Cold Spring Harb. Symp. Quant. Biol. 70, 177–185 (2005).
Google Scholar
-
Pearson, B. J. & Sanchez Alvarado, A. Regeneration, stem cells, and the evolution of tumor suppression. Cold Spring Harb. Symp. Quant. Biol. 73, 565–572 (2008).
Google Scholar
-
Pomerantz, J. H. & Blau, H. M. Tumor suppressors: enhancers or suppressors of regeneration? Development 140, 2502–2512 (2013).
Google Scholar
-
Brockes, J. P., Kumar, A. & Velloso, C. P. Regeneration as an evolutionary variable. J. Anat. 199, 3–11 (2001).
Google Scholar
-
Tanaka, E. M., Gann, A. A., Gates, P. B. & Brockes, J. P. Newt myotubes reenter the cell cycle by phosphorylation of the retinoblastoma protein. J. Cell Biol. 136, 155–165 (1997).
Google Scholar
-
Petersen, C. P. & Reddien, P. W. Wnt signaling and the polarity of the primary body axis. Cell 139, 1056–1068 (2009).
Google Scholar
-
Lovely, A. M. et al. Wnt signaling coordinates the expression of limb patterning genes during axolotl forelimb development and regeneration. Front. Cell Dev. Biol. 10, 814250 (2022).
Google Scholar
-
Driever, W. & Nusslein-Volhard, C. A gradient of bicoid protein in Drosophila embryos. Cell 54, 83–93 (1988).
Google Scholar
-
Driever, W. & Nusslein-Volhard, C. The bicoid protein determines position in the Drosophila embryo in a concentration-dependent manner. Cell 54, 95–104 (1988).
Google Scholar
-
Namba, R., Pazdera, T. M., Cerrone, R. L. & Minden, J. S. Drosophila embryonic pattern repair: how embryos respond to bicoid dosage alteration. Development 124, 1393–1403 (1997).
Google Scholar
-
Guerin, D. J., Kha, C. X. & Tseng, K. A. From cell death to regeneration: rebuilding after injury. Front. Cell Dev. Biol. 9, 655048 (2021).
Google Scholar
-
Wolpert, L. Positional information and the spatial pattern of cellular differentiation. J. Theor. Biol. 25, 1–47 (1969).
Google Scholar
-
Schad, E. G. & Petersen, C. P. STRIPAK limits stem cell differentiation of a WNT signaling center to control planarian axis scaling. Curr. Biol. 30, 254–263.e252 (2020).
Google Scholar
-
Brandao, A. S. et al. A regeneration-triggered metabolic adaptation is necessary for cell identity transitions and cell cycle re-entry to support blastema formation and bone regeneration. Elife 11, e76987 (2022).
-
Coffman, J. A. & Su, Y. H. Redox regulation of development and regeneration. Curr. Opin. Genet. Dev. 57, 9–15 (2019).
Google Scholar
-
Mammoto, T. & Ingber, D. E. Mechanical control of tissue and organ development. Development 137, 1407–1420 (2010).
Google Scholar
-
Nelson, C. M. Mechanical control of cell differentiation: insights from the early embryo. Annu. Rev. Biomed. Eng. 24, 307–322 (2022).
Google Scholar
-
McLaughlin, K. A. & Levin, M. Bioelectric signaling in regeneration: mechanisms of ionic controls of growth and form. Dev. Biol. 433, 177–189 (2018).
Google Scholar
-
El-Sherif, E. & Levine, M. Shadow enhancers mediate dynamic shifts of gap gene expression in the drosophila embryo. Curr. Biol. 26, 1164–1169 (2016).
Google Scholar
-
Farley, E. K. et al. Suboptimization of developmental enhancers. Science 350, 325–328 (2015).
Google Scholar
-
Hnisz, D. et al. Super-enhancers in the control of cell identity and disease. Cell 155, 934–947 (2013).
Google Scholar
-
Lettice, L. A. et al. A long-range Shh enhancer regulates expression in the developing limb and fin and is associated with preaxial polydactyly. Hum. Mol. Genet. 12, 1725–1735 (2003).
Google Scholar
-
Prescott, S. L. et al. Enhancer divergence and cis-regulatory evolution in the human and chimp neural crest. Cell 163, 68–83 (2015).
Google Scholar
-
Fausett, B. V., Gumerson, J. D. & Goldman, D. The proneural basic helix-loop-helix gene ascl1a is required for retina regeneration. J. Neurosci. 28, 1109–1117 (2008).
Google Scholar
-
Yurco, P. & Cameron, D. A. Cellular correlates of proneural and Notch-delta gene expression in the regenerating zebrafish retina. Vis. Neurosci. 24, 437–443 (2007).
Google Scholar
-
Akagi, T. et al. Requirement of multiple basic helix-loop-helix genes for retinal neuronal subtype specification. J. Biol. Chem. 279, 28492–28498 (2004).
Google Scholar
-
Tomita, K., Nakanishi, S., Guillemot, F. & Kageyama, R. Mash1 promotes neuronal differentiation in the retina. Genes Cells 1, 765–774 (1996).
Google Scholar
-
Elsaeidi, F. et al. Notch suppression collaborates with Ascl1 and Lin28 to unleash a regenerative response in fish retina, but not in mice. J. Neurosci. 38, 2246–2261 (2018).
Google Scholar
-
Karl, M. O. et al. Stimulation of neural regeneration in the mouse retina. Proc. Natl. Acad. Sci. USA 105, 19508–19513 (2008).
Google Scholar
-
Pollak, J. et al. ASCL1 reprograms mouse Muller glia into neurogenic retinal progenitors. Development 140, 2619–2631 (2013).
Google Scholar
-
Jorstad, N. L. et al. Stimulation of functional neuronal regeneration from Muller glia in adult mice. Nature 548, 103–107 (2017).
Google Scholar
-
Yan, R. et al. An enhancer-based gene-therapy strategy for spatiotemporal control of cargoes during tissue repair. Cell Stem Cell 30, 96–111.e116 (2023).
Google Scholar
-
Fuentes, D. R., Swigut, T. & Wysocka, J. Systematic perturbation of retroviral LTRs reveals widespread long-range effects on human gene regulation. Elife 7, e35989 (2018).
-
Grow, E. J. et al. Intrinsic retroviral reactivation in human preimplantation embryos and pluripotent cells. Nature 522, 221–225 (2015).
Google Scholar
-
Macfarlan, T. S. et al. Embryonic stem cell potency fluctuates with endogenous retrovirus activity. Nature 487, 57–63 (2012).
Google Scholar
-
Sanchez Alvarado, A. Regeneration in the metazoans: why does it happen? Bioessays 22, 578–590 (2000).
Google Scholar
-
Sanchez Alvarado, A. To solve old problems, study new research organisms. Dev. Biol. 433, 111–114 (2018).
Google Scholar
-
Birnbaum, K. D. & Sanchez Alvarado, A. Slicing across kingdoms: regeneration in plants and animals. Cell 132, 697–710 (2008).
Google Scholar
-
Seifert, A. W. et al. The influence of fundamental traits on mechanisms controlling appendage regeneration. Biol. Rev. Camb. Philos. Soc. 87, 330–345 (2012).
Google Scholar
-
Saxena, S., Vekaria, H., Sullivan, P. G. & Seifert, A. W. Connective tissue fibroblasts from highly regenerative mammals are refractory to ROS-induced cellular senescence. Nat. Commun. 10, 4400 (2019).
Google Scholar
-
Seifert, A. W. & Voss, S. R. Revisiting the relationship between regenerative ability and aging. BMC Biol. 11, 2 (2013).
Google Scholar
-
Sandoval-Guzman, T. et al. Fundamental differences in dedifferentiation and stem cell recruitment during skeletal muscle regeneration in two salamander species. Cell Stem Cell 14, 174–187 (2014).
Google Scholar
-
Simon, A. Regenerative biology: on with their heads. Nature 500, 32–33 (2013).
Google Scholar
-
Stockdale, W. T. et al. Heart regeneration in the Mexican Cavefish. Cell Rep. 25, 1997–2007.e1997 (2018).
Google Scholar
-
Vila-Farré, M. et al. Probing the evolutionary dynamics of whole-body regeneration within planarian flatworms. bioRxiv, https://www.biorxiv.org/content/10.1101/2022.12.19.520916v1 (2022).
-
Munafo, M. R. & Davey Smith, G. Robust research needs many lines of evidence. Nature 553, 399–401 (2018).
Google Scholar
-
Imperadore, P., Jones, K. M., Morgan, J. R., De Sio, F. & Stahnisch, F. W. Editorial: regeneration from cells to limbs: past, present, and future. Front. Cell Dev. Biol. 11, 1229613 (2023).
Google Scholar
-
Hariharan, I. K. & Serras, F. Imaginal disc regeneration takes flight. Curr. Opin. Cell Biol. 48, 10–16 (2017).
Google Scholar
-
Ponomareva, L. V., Athippozhy, A., Thorson, J. S. & Voss, S. R. Using Ambystoma mexicanum (Mexican axolotl) embryos, chemical genetics, and microarray analysis to identify signaling pathways associated with tissue regeneration. Comp. Biochem. Physiol. C. Toxicol. Pharm. 178, 128–135 (2015).
Google Scholar
-
Sinclair, J. W. et al. The Warburg effect is necessary to promote glycosylation in the blastema during zebrafish tail regeneration. NPJ Regen. Med. 6, 55 (2021).
Google Scholar
-
Goss, R. J. & Holt, R. Epimorphic vs. tissue regeneration in Xenopus forelimbs. J. Exp. Zool. 261, 451–457 (1992).
Google Scholar
-
Korneluk, R. G. & Liversage, R. A. Tissue regeneration in the amputated forelimb of Xenopus laevis froglets. Can. J. Zool. 62, 2383–2391 (1984).
Google Scholar
-
Agata, K., Saito, Y. & Nakajima, E. Unifying principles of regeneration I: epimorphosis versus morphallaxis. Dev. Growth Differ. 49, 73–78 (2007).
Google Scholar
-
Reddien, P. W. & Alvarado, A. S. Fundamentals of planarian regeneration. Annu. Rev. Cell Dev. Biol. 20, 725–757 (2004).
Google Scholar
-
Forsthoefel, D. J., Park, A. E. & Newmark, P. A. Stem cell-based growth, regeneration, and remodeling of the planarian intestine. Dev. Biol. 356, 445–459 (2011).
Google Scholar
-
Agata, K., Tanaka, T., Kobayashi, C., Kato, K. & Saitoh, Y. Intercalary regeneration in planarians. Dev. Dyn. 226, 308–316 (2003).
Google Scholar
-
Morgan, T. H. Experimental studies of the regeneration of Planaria maculata. Vol. 2 (W. Engelmann, 1898).
-
Tartar, V. The biology of stentor: a volume in international series of monographs in pure and applied biology. Division: Zoology. (Pergamon, 1961).
-
Goss, R. J. Principles of Regeneration. (Academic Press, 1969).
-
Hay, E. D. In: The stability of the differentiated state (ed. H. Ursprung) 85–108 (Springer Berlin Heidelberg, 1968).
-
Tata, P. R. & Rajagopal, J. Cellular plasticity: 1712 to the present day. Curr. Opin. Cell Biol. 43, 46–54 (2016).
Google Scholar
-
Gurdon, J. B. The developmental capacity of nuclei taken from intestinal epithelium cells of feeding tadpoles. Development 10, 622–640 (1962).
-
Gurdon, J. B. & Uehlinger, V. “Fertile” intestine nuclei. Nature 210, 1240–1241 (1966).
Google Scholar
-
Takahashi, K. & Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676 (2006).
Google Scholar
-
Waddington, C. H. An introduction to modern genetics. (The Macmillan Company, New York, 1939).
-
Levo, M. et al. Transcriptional coupling of distant regulatory genes in living embryos. Nature 605, 754–760 (2022).
Google Scholar
-
Long, H. K., Prescott, S. L. & Wysocka, J. Ever-changing landscapes: transcriptional enhancers in development and evolution. Cell 167, 1170–1187 (2016).
Google Scholar
-
Panigrahi, A. & O’Malley, B. W. Mechanisms of enhancer action: the known and the unknown. Genome Biol. 22, 108 (2021).
Google Scholar
-
Gehrke, A. R. et al. Acoel genome reveals the regulatory landscape of whole-body regeneration. Science 363, eaau6173 (2019).
-
Goldman, J. A. et al. Resolving heart regeneration by replacement histone profiling. Dev. Cell 40, 392–404.e395 (2017).
Google Scholar
-
Harris, R. E., Setiawan, L., Saul, J. & Hariharan, I. K. Localized epigenetic silencing of a damage-activated WNT enhancer limits regeneration in mature Drosophila imaginal discs. Elife 5, e11588 (2016).
-
Kang, J. et al. Modulation of tissue repair by regeneration enhancer elements. Nature 532, 201–206 (2016).
Google Scholar
-
Pronobis, M. I., Zheng, S., Singh, S. P., Goldman, J. A. & Poss, K. D. In vivo proximity labeling identifies cardiomyocyte protein networks during zebrafish heart regeneration. Elife 10,66079 (2021).
-
Sun, F. et al. Enhancer selection dictates gene expression responses in remote organs during tissue regeneration. Nat. Cell Biol. 24, 685–696 (2022).
Google Scholar
-
Wang, W. et al. Changes in regeneration-responsive enhancers shape regenerative capacities in vertebrates. Science 369, aaz3090 (2020).
-
Bonn, S. et al. Tissue-specific analysis of chromatin state identifies temporal signatures of enhancer activity during embryonic development. Nat. Genet. 44, 148–156 (2012).
Google Scholar
-
Rada-Iglesias, A. et al. A unique chromatin signature uncovers early developmental enhancers in humans. Nature 470, 279–283 (2011).
Google Scholar
-
Frankel, N. et al. Phenotypic robustness conferred by apparently redundant transcriptional enhancers. Nature 466, 490–493 (2010).
Google Scholar
-
Fulco, C. P. et al. Systematic mapping of functional enhancer-promoter connections with CRISPR interference. Science 354, 769–773 (2016).
Google Scholar
-
Osterwalder, M. et al. Enhancer redundancy provides phenotypic robustness in mammalian development. Nature 554, 239–243 (2018).
Google Scholar
-
Field, A. & Adelman, K. Evaluating enhancer function and transcription. Annu. Rev. Biochem. 89, 213–234 (2020).
Google Scholar
-
Martire, S. et al. Phosphorylation of histone H3.3 at serine 31 promotes p300 activity and enhancer acetylation. Nat. Genet. 51, 941–946 (2019).
Google Scholar
-
Neumayr, C. et al. Differential cofactor dependencies define distinct types of human enhancers. Nature 606, 406–413 (2022).
Google Scholar
Acknowledgements
We thank the Banbury Center for providing an amazing atmosphere for our vintage workshop experiment and, specifically, Rebecca Leshan for her backing and encouragement. We acknowledge Genentech and The Cold Spring Harbor Laboratory Corporate Sponsor Program for supporting the workshop. In addition to input from all attendees of the Banbury Workshop that informed this perspective article, we thank Francesca Mariani for significant editorial input. In a better-designed version of itself, academia would encourage the type of collaborative effort that went into this paper to be formally recognized with co-authorship. While nothing precludes this arrangement, how current conflict of interest (COI) declarations impact review procedures for grant proposals and promotion panels created unavoidable conflicts among participants to be co-authors. Lastly, we would like to thank John (Jack) Allen and Kelly Ross for their perspectives on an early version of this paper. Research in A.W. Seifert’s lab is funded by NIH grants R01 AR070313, R21 DE028070, the ASAP Collaborative Research Network through the Michael J. Fox Foundation, and DOD CDMRP 6W81XWH2110503. Research in R.M. Zayas’s lab is funded by NIH grant R01 GM135657. Research in E.M. Duncan’s lab is funded through NIH grant R35 GM142679.
Author information
Authors and Affiliations
Contributions
A.W.S., E.M.D., and R.M.Z. recorded detailed notes of discussions from the Banbury Workshop (Box 1). A.W.S., E.M.D., and R.M.Z. used the compiled notes to draft the manuscript. R.M.Z. incorporated suggested edits from meeting participants into the drafted manuscript. A.W.S. and E.M.D. created the illustrations with input from R.M.Z. A.W.S., E.M.D., and R.M.Z. revised the document and discussed all major revisions.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editors: Simona Chera and Anam Akhtar. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Peer Review File
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
Reprints and Permissions
About this article
Cite this article
Seifert, A.W., Duncan, E.M. & Zayas, R.M. Enduring questions in regenerative biology and the search for answers.
Commun Biol 6, 1139 (2023). https://doi.org/10.1038/s42003-023-05505-7
-
Received: 09 June 2023
-
Accepted: 25 October 2023
-
Published: 09 November 2023
-
DOI: https://doi.org/10.1038/s42003-023-05505-7
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.