Blood
A neck-sparing short stem shows significantly lower blood loss in total hip arthroplasty compared to a neck-resecting short stem
Abstract
Short stems are associated with a significantly lower blood loss (BL) compared to straight stems in total hip arthroplasty (THA). Different types of stems differ in design, fixation and level of femoral neck osteotomy. Therefore, we sought to evaluate the difference regarding the perioperative BL between two short stems with different designs in direct anterior approach (DAA). A total of 187 THA performed by a single surgeon were analysed. 107 patients received a neck-resecting (Group A) and 80 patients a neck-sparing short stem (Group B). Blood counts of the day before surgery and of two days after surgery were evaluated. Total blood volume and BL were calculated. Additionally, duration of surgery was analysed. The perioperative BL was significantly lower in Group B (451.4 ± 188.4 ml) compared to Group A (546.6 ± 232.7 ml; p = 0.002). The postoperative haematocrit (31.6 ± 3.7% vs. 30.4 ± 4.4%; p = 0.049) and haemoglobin-level (11.0 ± 1.3 g/dL vs. 10.4 ± 1.5 g/dL; p = 0.002) were significantly higher in Group B. Duration of surgery was significantly shorter in Group B (62.0 ± 11.4 min vs. 72.6 ± 21.8 min; p < 0.001). The use of a neck-sparing short stem leads to a significantly decreased BL compared to a neck-resecting short stem in DAA THA. A less extensively conducted capsular release necessary for optimal femoral exposition might lead to a lower perioperative BL and shorter durations of surgery.
Introduction
Minimally invasive (MIS) approaches have been introduced in recent years in total hip arthroplasty (THA)1. Due to their geometry, standard straight stems require more exposition and resection of the proximal femur for an optimal component placement compared to modern generation short stems2. Additionally, short stems allow smaller skin incisions with less soft tissue damage, while still enabling an accurate reconstruction of the pre-arthritic hip-anatomy3,4,5. While short stems in general are quite heterogenous with different designs and fixation philosophies, some modern “calcar loading” short stems like the Optimys Stem (Mathys Ltd. Bettlach, Switzerland), the NANOS Stem (Smith&Nephew, Marl, Germany) or the ANA.NOVA proxy hip stem (ImplanTec GmbH, Moedling, Austria) aim for an individual restoration of pre-arthritic hip biomechanics with even lower bone loss using a femoral neck sparing design6,7. In comparison to neck-resecting short stems, the femoral neck osteotomy is conducted further proximally and can be slightly varied depending on the patient’s anatomy, which potentially allows for a more individual restoration of the proximal femur’s anatomy and a more physiological load distribution8. However, analysis of the individual anatomy of each patient and exact preoperative templating is crucial when performing THA using a neck-sparing short stem as the restoration of the pre-arthritic hip biomechanics strongly depends on a precisely conducted femoral neck osteotomy as it significantly influences stem position and consequently hip-biomechanics—especially the hip offset6.
Although the differentiation between the effect of the stem itself on the perioperative blood loss (BL) and the approach it is implanted through is difficult, the use of short stems also seems to enable lower levels of perioperative BL and lower rates of blood transfusions compared to the use of straight-stems9. Nonetheless, perioperative BL is inevitable when performing THA. Reports of overall BL associated with THA range from 540 to 1600 ml depending on different factors like approach, implant, duration of surgery and perioperative blood loss prophylaxis9,10,11.
With an increasing socio-economic burden on health care providers, rising numbers of outpatient THA and in general increasing trends towards early mobilization and early discharge from hospital, optimization of the perioperative management becomes more and more important12,13,14,15. One contributing factor to postoperative pain, swelling and possibly delayed mobilization is the perioperative BL and consecutive hematomas, which are to some extent inevitable after THA16,17. Furthermore, a lower perioperative BL might positively influence the overall outcome, as blood transfusions after THA seem to be associated with higher rates of periprosthetic infections, complications and longer length of stay at the hospital18,19,20.
Up to date there are hardly reports regarding differences in BL between short stems with different designs and different fixation philosophies requiring different levels of femoral neck resection. Therefore, we sought to evaluate possible impacts of stem design and level of femoral neck resection on the perioperative BL associated with THA.
Materials and methods
Study population
A consecutive series of 254 hips with unilateral index surgery between January 1st 2017 and July 31th 2022 operated by a single surgeon using a MIS direct anterior approach (DAA) to the hip were retrospectively screened for inclusion. The medical records until discharge from hospital were evaluated. In 159 of the cases the Fitmore hip stem (ZimmerBiomet, Warsaw, IN, USA) combined with the Allofit/-S press-fit acetabular cup (ZimmerBiomet, Warsaw, IN, USA) (Group A) and in 95 of the cases the ANA.NOVA proxy hip stem (ImplanTec GmbH, Moedling, Austria) combined with the ANA.NOVA Alpha acetabular cup (ImplanTec GmbH, Moedling, Austria) (Group B) were implanted.
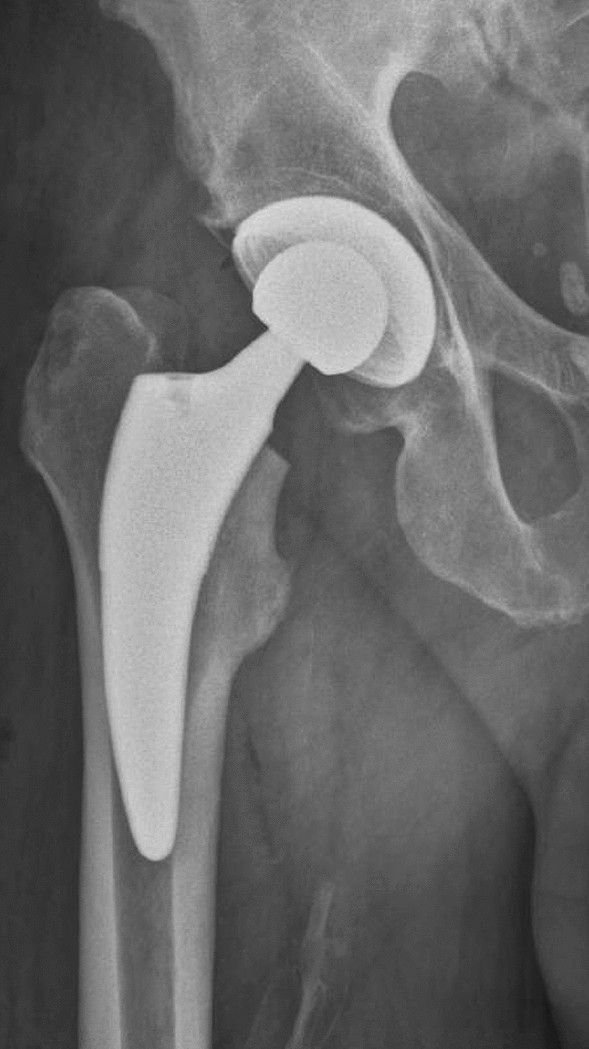
The cementless titanium alloy (TiAl6V4) Fitmore hip stem features a porolock Ti-VPS coating in the proximal part for enhanced bone ingrowth. It is available in four different neck angle options and in 14 different sizes for each offset option2,21. To achieve press-fit fixation, the stem has a triple tapered design. It can be classified as neck-harming short stem according to the recommended level of resection of the femoral neck (Fig. 1)21,22. Reports available in the literature show excellent clinical performance of this stem with high patient satisfaction and high survival rates of 93.7% for revision for all causes and 99.6% for revision due to aseptic loosening at a follow up of 8.6 years2,21,23.
Shows the neck-resecting Fitmore hip stem and press-fit acetabular cup used within Group A.
The cementless titanium alloy (TiAl6V4) ANA.NOVA proxy hip stem has a rough titanium plasma coating with electrochemically applied hydroxyapatite (BONIT) to enhance osteointegration. It features a triple tapered design with a calcar guided press fit fixation with a 3-point anchorage with the main fixation zone between medial calcar and lateral cortex. It is available in 12 different sizes with two offset options for each size24. It can be classified as partial femoral neck-sparing short stem according to the recommended level of resection of the femoral neck (Fig. 2)22. Up to date, there are hardly reports regarding the clinical performance and survival rate of this stem. However, it seems to enable satisfying hip geometry restoration and low revision rates due to subsidence at a follow up of 3 years24,25.

Shows the neck-sparing ANA.NOVA proxy hip stem and press-fit acetabular cup used within Group B.
The medical records of the patients were screened primarily for laboratory results regarding perioperative BL. Patients with systematic diseases affecting the blood count and lacking perioperative and postoperative laboratory results were excluded from this study. The first 50 cases performed via DAA in combination with the Fitmore hip stem were defined as learning curve for the approach itself and were therefore excluded from the study26,27,28. Further exclusion criteria were any other approaches to the hip apart from the DAA as well as the occurrence of any intraoperative complications such as fractures.
The study was approved by the ethics committee of the medical faculty of the Johannes Kepler University Linz (Reference number: 1140/2022). Due to the retrospective study design with evaluation of pre-existing medical records the need for informed consent was waived by the ethics committee of the medical faculty of the Johannes Kepler University Linz. All procedures performed were in accordance with the principles of the Declaration of Helsinki.
Surgical technique and postoperative treatment protocol
In all cases the standardized peri- and postoperative protocol was identical. Tranexamic acid (20 mg per kilogram of body weight) was administered routinely prior to skin incision. Surgical procedures were performed by a single fellowship-trained consultant. In all cases a minimally invasive DAA without the use of a traction table was performed as previously described29. Components were implanted according to the manufacturer’s instructions and aiming for a restoration of the pre-arthritic biomechanics of the affected hip. Local infiltration anaesthesia (0.5 mg Epinephrin and 1 mg Ropivacaine per 100 ml Sodium chloride) was performed in all cases prior to wound closure. Weight-bearing was tolerated immediately after surgery. In case of a postoperative haemoglobin level below 8 g/dL, blood transfusion was conducted if clinical symptoms of anaemia were present. In case of a postoperative haemoglobin level below 7 g/dL, blood transfusion was conducted regardless of clinical symptoms of anaemia.
Medical record evaluation and blood loss calculation
The medical record of each patient was screened for laboratory results in order to calculate the perioperative BL. The blood count of the day of admission to the surgical unit, which was scheduled one day prior to surgery, was analysed regarding Haematocrit- and Haemoglobin-Level. Laboratory results of the same parameters were analysed for the second day after surgery as well. The patients’ estimated blood volume (BV) was calculated using the formula described by Nadler et al.30: (BV male patients=604+0.0003668 times left[{sizeleft(cmright)}^{3}right]+32.2 times weightleft(kgright);BV female patients=183+0.000356times left[{sizeleft(cmright)}^{3}right]+33times weightleft(kgright).) The estimated perioperative BL was calculated using a modified version of the formula described by Mercuriali et al.31 using the Haematocrit level measured on the second day after surgery instead of the Haematocrit level measured on the fifth day after surgery as described by Mercuriali et al.31 (Estimated BL=BV times {(Hct}_{preoperative}-{Hct}_{2 days postoperative})+ml of transfused blood). Blood transfusions within this time period were evaluated and taken into account when calculating the estimated BL. Additional parameters such as patient specific data like gender, age, height, weight, BMI and ASA-Score as well as surgery specific data like component sizes and duration of surgery and length of stay at the hospital were analysed too.
Statistical analysis
Statistical analysis was performed using SPSS version 28 (IBM SPSS statistics, Chicago, IL, USA). Arithmetic mean value and standard deviation were calculated for metric scaled data. Kolmogorov–Smirnov-Test was performed to test for normal distribution. For normally distributed parameters Chi-Square-Test was performed to analyse categorial parameters while t-Test was performed to analyse metric scaled parameters. For non-normally distributed metric parameters Man-Whitney-U-Test was conducted. A p value < 0.05 was considered as statistically significant.
Results
In total 187 patients were included for analysis in the present study. A total of 4 intraoperative fractures with 2 cases in each study group (p = 0.574) occurred within the study population (Group A: 1 intraoperative acetabular fissure and 1 intraoperative fracture of the greater trochanter—both treated conservatively; Group B: 1 intraoperative femoral shaft fracture treated with a revision stem and cerclages and 1 intraoperative fracture of the greater trochanter treated conservatively) and were excluded from the analysis (Fig. 3). 52.9% of the study population were female patients and the mean age was 67.8 ± 10.7 years within the study population (Table 1). There were no significant differences regarding the preoperative calculated BV, Haematocrit- or Haemoglobin-levels between the two study groups (Table 2). Postoperatively, the calculated BL of Group B (451.4 ± 188.4 ml) was significantly lower compared to Group A (546.6 ± 232.7 ml; p = 0.002). The haematocrit (Group A: 30.4 ± 4.4%; Group B: 31.6 ± 3.7%; p = 0.049) and the haemoglobin-level (Group A: 10.4 ± 1.5 g/dL; Group B: 11.0 ± 1.3 g/dL; p = 0.001) at the second day after surgery were significantly lower within Group A. Blood transfusions were administered significantly less often in Group B (1.2%) compared to Group A (9.3%; p = 0.025). The average duration of the surgery was significantly shorter within Group B (62.0 ± 11.4 min) in relation to Group A (72.6 ± 21.8 min; p < 0.001). Overall length of stay at the hospital was significantly shorter within Group B (6.1 ± 1.7 days) compared to Group A (6.8 ± 2.9 days; p = 0.029).

Shows the formation of the two study subgroups depending on the implant used for Total Hip Arthroplasty (THA) performed via Direct Anterior Approach (DAA); Group A: neck-resecting Fitmore hip stem; Group B: neck-sparing ANA.NOVA proxy hip stem.
Discussion
The results of this study reveal a significantly lower BL of a partially neck-sparing short stem compared to a neck-resecting short stem. Additionally, the average duration of the surgery as well as the length of stay at the hospital were significantly shorter for THA using a neck-sparing stem with epi-metaphyseal fixation.
Up to date, there are hardly reports investigating the differences in perioperative BL between different types of hip stems. While previous studies showed lower rates of perioperative BL comparing short stems to straight stems implanted through different approaches, this is the first study investigating the differences in perioperative BL between two different types of short stems implanted by a single surgeon through the same standardized MIS DAA without traction table and the same perioperative treatment protocol for all patients9.
A total of 4 intraoperative fractures occurred within the study population with no significant difference between the two study groups (2 intraoperative fractures in each study group; p = 0.574). Those cases were excluded from the analysis to avoid distortion of the study’s findings as intraoperative fractures potentially significantly influence the perioperative BL as well as the duration of surgery. However, intraoperative and early postoperative femoral fractures are well-known complications associated with DAA32. The overall observed rate of 2.1% of intraoperative fractures within this study’s population is matching the findings of other reports33,34,35.
There are several factors influencing BL during hip surgery that must be considered. Within this study, a MIS DAA was performed in all cases, which seems to be associated with less BL compared to other approaches like a lateral or a posterior approach, which might be caused by a tendentially shorter skin incision and comparatively less soft tissue damage36,37. Tranexamic acid was administered routinely prior to skin incision by the anaesthetist within this study, which also seems to reduce BL following THA and also reduce the postoperative rate of blood transfusions after THA38,39. Additionally, local infiltration anaesthesia was performed in all patients prior to wound closure, which also seems to reduce BL after joint arthroplasty40,41. Within this study, no surgical drains were applied after THA, which seems to have benefits regarding minimizing the perioperative BL respectively the transfusion rate after THA as well42,43. In general, the calculated BL within this study is low compared to other reports investigating BL after THA via DAA39. This might be due to the modification of the formula described by Mercuriali et al.31 in form of evaluating the postoperative haematocrit level of the second day after surgery instead of the haematocrit level of the fifth day after surgery, as other reports evaluated blood counts taken at a later postoperative stage9. While still being mostly in line with other reports investigating the BL after THA within the first three postoperative days, the calculation of the absolute value of the perioperative BL after THA was less of an objective of this study than comparing the differences in perioperative BL between the two study groups10.
Duration of surgery also seems to be associated with BL during THA, which might be one contributing factor regarding the lower calculated BL within Group B of this study, as the duration of surgery was significantly lower in Group B compared to Group A42. However, the other main factor associated with lower BL within this study might be the partially femoral neck-sparing short stem with epi-metaphyseal fixation used within Group B. In general, short stems seem to enable THA with tendentially lower amounts of perioperatively BL when compared to straight stems9. In theory, the femoral stem used within Group B of this study combined with the DAA required a more proximally conducted femoral osteotomy due it’s fixation philosophy which tendentially led to a more upright angle of the surface of the osteotomy and therefore allowed an easier achievement of an optimal exposition of the femoral neck for implanting the stem. Additionally, the easier exposition of the proximal femur might have allowed for a less extensively conducted capsular release, which on one hand—due to the blood vessels surrounding the femoral neck—might have contributed to the lower BL within Group B and on the other hand also might have contributed to the on average significantly lower duration of surgery within this Group44.
Length of stay at the hospital on average was significantly shorter within patients who received the femoral stem with epi-metaphyseal fixation compared to those who received the stem with metaphyseal fixation (6.1 days vs. 6.8 days; p = 0.029). As for that matter, the perioperative BL consecutive hematoma and pain might have also had an influence on the length of stay at the hospital after THA, as other parameters like duration of the surgery, patient age or BMI seem not to necessarily influence length of stay at the hospital45.
However, there are some limitations to this study that must be kept in mind when interpreting the findings of this study. Firstly, this is a single centre retrospective cohort study with a single surgeon setting. Moreover, due to the design of the present study, no randomization was performed, as the surgeon chose which implant to use for each surgery. For example, patients with certain anatomical characteristics like Dorr-Type-C femora or valgus hips were less likely to receive a neck-sparing short stem within the first few cases, although over the further course of this study there was no contraindication for using a neck-sparing short stem due to certain anatomical characteristics with exception of severe hip dysplasia (Crowe > 1). Nevertheless, there is a chance of selection bias, which represents another limitation of this study. Additionally, estimated BL was calculated using the blood count of the second day after surgery, which also limits the results of this study, as blood counts from for example five days after surgery could have provided additional insights. However, a reasonable evaluation of postoperative blood counts other than on the second day after surgery were not possible within this study as many patients—to some extent caused by limits of capacity and infectiological reasons during the COVID-19 pandemic—were discharged from hospital before the fifth day after surgery without undergoing another blood sample. Therefore, evaluation of subsequent blood samples would have led to a considerably higher rate of patients lost to follow-up.
In summary, the use of a neck-sparing short stem leads to a significantly decreased BL in DAA compared to a neck-resecting short stem. A less extensively conducted capsular release necessary for optimal femoral exposition might lead to a lower perioperative BL with shorter durations of surgery. Therefore, the use of a neck sparing short stem can be recommended when performing DAA. However, further evaluations with bigger study populations are necessary to proof these findings.
Data availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
References
-
Learmonth, I. D., Young, C. & Rorabeck, C. The operation of the century: total hip replacement. Lancet 370, 1508–1519 (2007).
Google Scholar
-
Gustke, K. Short stems for total hip arthroplasty. J. Bone Joint Surg. Br. 94-B, 47–51 (2012).
-
Brun, O.-C.L., Sund, H. N., Nordsletten, L., Röhrl, S. M. & Mjaaland, K. E. Component placement in direct lateral versus minimally invasive anterior approach in total hip arthroplasty: Radiographic outcomes from a prospective randomized controlled trial. J. Arthroplasty 34, 1718–1722 (2019).
Google Scholar
-
Migliorini, F. et al. Total hip arthroplasty: Minimally invasive surgery or not? Meta-analysis of clinical trials. Int. Orthop. 43, 1573–1582 (2019).
Google Scholar
-
von Roth, P. et al. Reproducibility of femoral offset following short stem and straight stem total hip arthroplasty. Orthopedics 37, e678–e684 (2014).
-
Kutzner, K. Calcar-guided short-stem total hip arthroplasty: Will it be the future standard? Review and perspectives. World J. Orthop. 12, 534–547 (2021).
Google Scholar
-
Khanuja, H. S., Banerjee, S., Jain, D., Pivec, R. & Mont, M. A. Short bone-conserving stems in cementless hip arthroplasty. JBJS 96(20), 1742–1752 (2014).
-
Sivaloganathan, S., Maillot, C., Harman, C., Villet, L. & Rivière, C. Neck-sparing short femoral stems: A meta-analysis. Orthop. Traumatol. Surg. Res. 106, 1481–1494 (2020).
Google Scholar
-
Hochreiter, J., Hejkrlik, W., Emmanuel, K., Hitzl, W. & Ortmaier, R. Blood loss and transfusion rate in short stem hip arthroplasty. A comparative study. Int. Orthop. 41, 1347–1353 (2017).
Google Scholar
-
Mortazavi, S. M. J. et al. The efficacy of bone wax in reduction of perioperative blood loss in total hip arthroplasty via direct anterior approach: A prospective randomized clinical trial. JBJS 104, 1805–1813 (2022).
-
Meermans, G., Konan, S., Das, R., Volpin, A. & Haddad, F. S. The direct anterior approach in total hip arthroplasty. Bone Jt. J. 99-B, 732–740 (2017).
Google Scholar
-
Klug, A. et al. Future burden of primary and revision hip arthroplasty in Germany: A socio-economic challenge. Arch. Orthop. Trauma Surg. 141, 2001–2010 (2021).
Google Scholar
-
Pollock, M., Somerville, L., Firth, A. & Lanting, B. Outpatient total hip arthroplasty, total knee arthroplasty, and unicompartmental knee arthroplasty: A systematic review of the literature. JBJS Rev. 4, e4 (2016).
Google Scholar
-
DeMik, D. E. et al. Recent increases in outpatient total hip arthroplasty have not increased early complications. J. Arthroplasty 37, 325-329.e1 (2022).
Google Scholar
-
Tanzer, D., Smith CRA, K. & Tanzer, M. Changing patient expectations decreases length of stay in an enhanced recovery program for THA. Clin. Orthop. Relat. Res. 476, 372–378 (2018).
Google Scholar
-
Fernández Palomo, L. J., González Pola, R. & Castillo Vázquez, F. G. Iliopsoas hematoma after total hip arthroplasty using a minimally invasive modified direct anterior approach: A case report. JBJS Case Connect. 12(1), e21 (2022).
-
Zeng, W. et al. Comparison between drainage and non-drainage after total hip arthroplasty in chinese subjects. Orthop. Surg. 6, 28–32 (2014).
Google Scholar
-
Friedman, R., Homering, M., Holberg, G. & Berkowitz, S. D. Allogeneic blood transfusions and postoperative infections after total hip or knee arthroplasty. JBJS 96, 272–278 (2014).
-
Bou Monsef, J. & Boettner, F. Blood management may have an impact on length of stay after total hip arthroplasty. HSS J. 10, 124–130 (2014).
Google Scholar
-
Klasan, A. et al. Transfusions increase complications and infections after hip and knee arthroplasty: An analysis of 2760 cases. Technol. Health Care 26, 825–832 (2018).
Google Scholar
-
Innmann, M. M. et al. Fifty-six percent of proximal femoral cortical hypertrophies 6 to 10 years after Total hip arthroplasty with a short Cementless curved hip stem—A cause for concern?. BMC Musculoskelet. Disord. 20, 261 (2019).
Google Scholar
-
Oldenrijk, J., Molleman, J., Klaver, M., Poolman, R. & Haverkamp, D. Revision rate after short-stem total hip arthroplasty. Acta Orthop. 85, 250–258 (2014).
Google Scholar
-
Luger, M. et al. Low rate of early periprosthetic fractures in cementless short-stem total hip arthroplasty using a minimally invasive anterolateral approach. J. Orthop. Traumatol. 22, 19 (2021).
Google Scholar
-
Reinbacher, P. et al. Three-year migration analysis of a new metaphyseal anchoring short femoral stem in THA using EBRA-FCA. Sci. Rep. 12, 17173 (2022).
Google Scholar
-
Maurer-Ertl, W. et al. Restoration of hip geometry after total hip arthroplasty: retrospective comparison of two short stems and one straight stem. BMC Musculoskelet. Disord. 23, 1035 (2022).
Google Scholar
-
Garbarino, L. et al. Does structured postgraduate training affect the learning curve in direct anterior total hip arthroplasty? A single surgeon’s first 200 cases. Arthroplasty Today 7, 98–104 (2021).
Google Scholar
-
Li, Y.-W. et al. First 100 total hip arthroplasties performed by a young surgeon using the direct anterior approach: learning curve and complications. Arch. Orthop. Trauma Surg. https://doi.org/10.1007/s00402-023-05077-5 (2023).
Google Scholar
-
Metzger, C. M., Farooq, H., Hur, J. O. & Hur, J. Transitioning from the posterior approach to the direct anterior approach for total hip arthroplasty. Hip Pelvis 34, 203–210 (2022).
Google Scholar
-
Rachbauer, F. Minimally invasive total hip arthroplasty via direct anterior approach. Orthopade 34, 1103–1111 (2005).
Google Scholar
-
Nadler, S. B., Hidalgo, J. & Bloch, T. Prediction of blood volume in normal human adults. Surgery 51(2), 224–232 (1962).
Google Scholar
-
Mercuriali, F. & Inghilleri, G. Proposal of an algorithm to help the choice of the best transfusion strategy. Curr. Med. Res. Opin. 13, 465–478 (1996).
Google Scholar
-
Flevas, D. A., Tsantes, A. G. & Mavrogenis, A. F. Direct anterior approach total hip arthroplasty revisited. JBJS Rev. 8(4), e0144 (2020).
Google Scholar
-
Goyal, T., Choudhury, A. K., Paul, S., Das, L. & Gupta, T. The direct anterior approach without traction table: How does it compare with the posterior approach?—A prospective non-randomised trial. J. Clin. Orthop. Trauma 31, 101924 (2022).
Google Scholar
-
Jewett, B. A. & Collis, D. K. High complication rate with anterior total hip arthroplasties on a fracture table. Clin. Orthop. Relat. Res. 469, 503–507 (2011).
Google Scholar
-
Dietrich, M., Kabelitz, M., Dora, C. & Zingg, P. O. Perioperative fractures in cementless total hip arthroplasty using the direct anterior minimally invasive approach: Reduced risk with short stems. J. Arthroplasty 33, 548–554 (2018).
Google Scholar
-
Wang, Z. et al. A systematic review and meta-analysis of direct anterior approach versus posterior approach in total hip arthroplasty. J. Orthop. Surg. 13, 229 (2018).
-
Huang, X., Liu, D., Jia, B. & Xu, Y. Comparisons between direct anterior approach and lateral approach for primary total hip arthroplasty in postoperative orthopaedic complications: A systematic review and meta-analysis. Orthop. Surg. 13, 1707–1720 (2021).
Google Scholar
-
Gianakos, A. L., Hurley, E. T., Haring, R. S., Yoon, R. S. & Liporace, F. A. Reduction of blood loss by tranexamic acid following total hip and knee arthroplasty: A meta-analysis. JBJS Rev. 6, e1 (2018).
Google Scholar
-
Zhao, H. et al. Efficacy of oral tranexamic acid on blood loss in primary total hip arthroplasty using a direct anterior approach: a prospective randomized controlled trial. Int. Orthop. 42, 2535–2542 (2018).
Google Scholar
-
Pandazi, A. et al. Periarticular infiltration for pain relief after total hip arthroplasty: A comparison with epidural and PCA analgesia. Arch. Orthop. Trauma Surg. 133, 1607–1612 (2013).
Google Scholar
-
Bhutta, M. A., Ajwani, S. H., Shepard, G. J. & Ryan, W. G. Reduced blood loss and transfusion rates: Additional benefits of local infiltration anaesthesia in knee arthroplasty patients. J. Arthroplasty 30, 2034–2037 (2015).
Google Scholar
-
Huang, Z. et al. Analysis of a large data set to identify predictors of blood transfusion in primary total hip and knee arthroplasty. Transfusion 58, 1855–1862 (2018).
Google Scholar
-
Pempe, C. et al. Predictors for blood loss and transfusion frequency to guide blood saving programs in primary knee- and hip-arthroplasty. Sci. Rep. 11, 4386 (2021).
Google Scholar
-
Gautier, E., Ganz, K., Krügel, N., Gill, T. & Ganz, R. Anatomy of the medial femoral circumflex artery and its surgical implications. J. Bone Joint Surg. Br. 82, 679–683 (2000).
Google Scholar
-
Petis, S. M., Howard, J. L., Lanting, B. A., Somerville, L. E. & Vasarhelyi, E. M. Perioperative predictors of length of stay after total hip arthroplasty. J. Arthroplasty 31, 1427–1430 (2016).
Google Scholar
Acknowledgements
The article processing charge was supported by Johannes Kepler Open Access Publishing Fund.
Author information
Authors and Affiliations
Contributions
C.S.: original draft preparation, data acquisition, statistical analysis, data interpretation. B.S.: surgeon, draft review. K.B.: data acquisition. C.Sch.: data interpretation, draft review and editing. T.G.: draft review and editing. M.L.: conceptualization, data acquisition and interpretation, draft review and editing, supervision.
Corresponding author
Ethics declarations
Competing interests
Two of the authors declare the following Competing Interests: Bernhard Schauer declares the following Competing Interests: ImplanTec: Personal fees. Tobias Gotterbarm declares the following Competing Interests: Zimmer Biomet Europe: Grant, personal fees; Depuy Synthes Gmbh: Grant, personal fees; Mathys AG: Grant, personal fees; Medacta: Personal fees; ImplanTec: Personal fees. The other authors have no Competing Interests to declare.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
Reprints and Permissions
About this article
Cite this article
Stadler, C., Schauer, B., Brabec, K. et al. A neck-sparing short stem shows significantly lower blood loss in total hip arthroplasty compared to a neck-resecting short stem.
Sci Rep 13, 19695 (2023). https://doi.org/10.1038/s41598-023-47008-9
-
Received: 23 March 2023
-
Accepted: 07 November 2023
-
Published: 11 November 2023
-
DOI: https://doi.org/10.1038/s41598-023-47008-9
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.