Infection
Acinetobacter spp. bloodstream infection in hematological patients: a 10-year single-center study
Clinical characteristics and outcomes
Complete clinical data were obtained from 40 patients with Acinetobacter spp. BSI during the study period. The clinical characteristics of patients with Acinetobacter spp. BSI who survived or died within 30 days are summarised in Table 1. The sex distribution was similar between the groups, with a median age of 25.5 years (range: 1–62 years). The underlying diseases included very severe aplastic anaemia (VSAA), acute lymphoblastic leukaemia, acute myeloid leukaemia, myelodysplastic syndrome, and diffuse large B-cell lymphoma. During the same period, CRA colonization occurred in 25% (n = 10) of the patients with Acinetobacter spp. BSI. All patients developed fever, and 55.0% (n = 22) were diagnosed with pneumonia. 30% of the patients (n = 12) presented with pleural effusion (50.0% vs. 19.2%, P = 0.071), which accounted for a marginally higher proportion of non-survivors; 11 presented with oral mucositis or pharyngitis; two presented with sinus infections; four presented with skin or soft tissue infections; and six presented with perianal infections. In addition, five and nine patients developed an intracranial infection and respiratory failure, respectively. 20% (n = 10) of the patients with Acinetobacter spp. BSI eventually developed severe sepsis or septic shock.
The median length of hospital stay was 35 days. Non-survivors had a marginally longer median length of hospital stay (median: 21, [IQR: 8–36] days vs. median: 12, [IQR: 5–19] days; P = 0.118). Seven of the 40 patients died within 7 days, with a mortality rate of 25% at 14 days. By day 30, after the onset of bacteremia, treatment was ineffective in 37.5% (n = 15) of the patients. Furthermore, the 30-day all-cause mortality rate among patients with Acinetobacter spp. bloodstream infections (BSI) was as high as 35%. The 90-day mortality rate stood at 42.5%.
Antimicrobial therapy
All patients received empirical antibiotic therapy immediately after collecting blood culture samples. None of the patients used antibiotics for prophylaxis. Previous antibiotic use (92.9% vs. 80.8%, P = 0.399) and antimicrobial exposure at the onset of BSI (64.3% vs. 46.2%, P = 0.273) were not associated with 30-day mortality (Table 1). Moreover, the proportion of patients receiving appropriate empirical antibiotics differed markedly between non-survivors and survivors (15.4% vs. 57.1%, P = 0.011). A total of 52.5% (n = 21) of patients received definitive combination therapy, with no noticeable effect on 30-day survival (50.0% vs. 57.1%, P = 0.666).
The following data were analysed to understand the influence of appropriate empirical antibiotic therapy and combined target antibiotics on patients (Supplementary Table 1). Patients with CRA (91.7% vs. 17.9%, P < 0.001) and MDR Acinetobacter spp. (MDRA) (100.0% vs. 25.0%, P < 0.001) bacteremia often received inappropriate empirical antibiotic therapy within 24 h, and the definite therapy tended to be in combination (CRA: 61.9% vs. 15.8%, P = 0.003; MDRA: 66.7% vs. 26.3%, P = 0.011). Patients who received appropriate empirical antibiotic therapy had significantly higher microbial cure rates (57.1% vs. 8.3%, P = 0.004) and lower mortality rates (21.4% vs. 66.7%, P = 0.011) within 30 days.
Antimicrobial resistance of the isolates
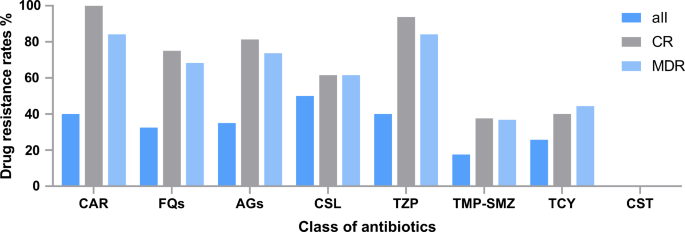
Antimicrobial resistance of Acinetobacter spp. isolates from the bloodstream is shown in Table 2. Of the 40 isolated strains, 40% (n = 16) were CRA, 47.5% (n = 19) were MDRA, and 25.8% (n = 8) were tetracycline-resistant Acinetobacter spp. Colistin exhibited the highest antimicrobial activity against the isolated strains (100.0%), followed by minocycline (92.3%), doxycycline (90.0%), trimethoprim-sulfamethoxazole (TMP-SMZ) (82.5%) and tigecycline (80.6%). Figure 1 shows the drug resistance of Acinetobacter spp. isolates to eight classes of antibiotics according to CR and MDR stratifications. The rates of resistance to carbapenems, fluoroquinolones, aminoglycosides, cefoperazone-sulbactam, piperacillin-tazobactam, TMP-SMZ, tetracyclines, and colistin of the CR strains were 100.0%, 75.0%, 81.3%, 61.5%, 93.8%, 37.5%, 40.0%, and 0.0%, respectively. In contrast, those of MDR strains were 84.2%, 68.4%, 73.7%, 61.5%, 84.2%, 36.8%, 44.4%, and 0.0%, respectively. For tetracycline-resistant Acinetobacter spp. BSI, TMP-SMZ and colistin were available. Furthermore, we have included the Minimum Inhibitory Concentrations for all 40 strains in tabular format (Supplementary Table 2).
Distribution of drug resistance according to carbapenem-resistant (CR) and multidrug-resistant (MDR) stratifications. CAR, carbapenems; FQs, fluoroquinolones; AGs, aminoglycosides; CSL, cefoperazone-sulbactam; TZP, piperacillin-tazobactam; TMP-SMZ, trimethoprim-sulfamethoxazole; TCY, tetracycline; CST, colistin
Risk factors for CRA BSI acquisition
In the univariate analysis, patients with hypoproteinaemia (68.8% vs. 34.8%, P = 0.037) and those with antimicrobial exposure at the onset of BSI (81.3% vs. 33.3%, P = 0.003) had an increased risk of developing CRA BSI (Table 3). Similarly, CRA colonization (62.5% vs. 0.0%, P < 0.001) and history of carbapenem use within 30 days (75.0% vs. 20.8%, P = 0.001) were factors associated with CRA BSI. In addition, a marginally larger proportion of patients had cardiac dysfunction (50.0% vs. 20.8%, P = 0.054). In the multivariate analysis, CRA colonization (Odds ratio (OR): 11.949, 95% CI: 1.799–79.363, P = 0.010) and previous exposure to carbapenems within 30 days (OR: 6.927, 95% CI: 1.125–42.638, P = 0.037) were independent risk factors for CRA BSI.
Risk factors for 30-day mortality in patients with Acinetobacter Spp. BSI
The prognostic factors associated with 30-day mortality were analysed after Acinetobacter spp. BSI with respect to hosts, pathogens, and treatments (Table 1). In the underlying disease, patients with VSAA had a higher 30-day mortality rate (42.9% vs. 11.5%, P = 0.044). The patients who died within 30 days were more likely to have intracranial infections (35.7% vs. 0.0%, P = 0.003), respiratory failure (57.1% vs. 3.8%, P < 0.001), cardiac dysfunction (57.1% vs. 19.2%, P = 0.031), and severe sepsis/septic shock (64.3% vs. 3.8%, P < 0.001). Most hematological patients (77.5%, n = 31) had neutropenia at the onset of Acinetobacter spp. BSI. Patients who died within 30 days usually had unresolved neutropenia for > 14 consecutive days, either before (58.3% vs. 24.0%, P = 0.067) or after infection (92.9% vs. 30.8%, P = 0.001). The non-survivors had significantly higher median PCT (median: 1.79 [IQR: 0.53–21.60] ug/L vs. median: 0.14 [IQR: 0.10–1.10] ug/L, P = 0.025) and CRP (median: 128.13 [IQR: 61.00–243.78] mg/L vs. median: 12.40 [IQR: 9.32–58.30] mg/L, P = 0.001) levels. These results suggest that these biomarkers are useful tools for assessing disease severity. Surprisingly, neither CRA (50.0% vs. 34.6%, P = 0.343) nor MDRA (57.1% vs. 42.3%, P = 0.370) were risk factors for 30-day mortality. Extensively drug-resistant Acinetobacter spp. (XDRA) (28.6% vs. 3.8%, P = 0.043) and tetracycline-resistant Acinetobacter spp. (55.6% vs. 13.6%, P = 0.027) were predictors of poor prognosis. In the multivariate model, inappropriate empirical antibiotic therapy was an independent risk factor for 30-day mortality (OR: 11.234, 95% CI: 1.261–20.086, P = 0.030).
The survival analysis showed that the 30-day survival probability of patients who received appropriate empirical antibiotic therapy was significantly higher than that of patients who received inappropriate empirical antibiotic therapy (78.6% [95% CI: 58.4–89.8%] vs. 33.3% [95% CI: 10.3–58.8%], P = 0.023) (Fig. 2A). Unresolved neutropenia after infection (38.1% [95% CI: 18.3–57.8%] vs. 94.7% [95% CI: 68.1–99.2%], P < 0.001) and tetracycline-resistant Acinetobacter spp. BSI (37.5% [95% CI: 8.7–67.4%] vs. 82.6% [95% CI: 60.1–93.1%], P = 0.019) were associated with worse survival (Fig. 2B and C). Furthermore, the 30-day survival probability of patients who experienced cardiac dysfunction was poorer than that of patients without cardiac dysfunction (38.5% [95% CI: 14.1–62.8%] vs. 77.8% [95% CI: 57.1–89.3%], P = 0.002) (Fig. 2D).

Kaplan-Meier curves of the 30-day probability of survival for patients with and without (A) appropriate empirical therapy, (B) resolved neutropenia after infection, (C) tetracycline-resistant Acinetobacter spp. BSI, and (D) cardiac dysfunction
Strain identification
This study used the VITEK 2 Compact to identify strains, most classified as A. baumannii or A. calcoaceticus-baumannii complex (Supplementary Table 3). Thirteen frozen CRA strains isolated from the bloodstream were resuscitated and identified, including A. pittii (n = 6), A. baumannii (n = 5), A. calcoaceticus (n = 1), and A. nosocomialis (n = 1) using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS). However, the WGS results reclassified the strain of A. calcoaceticus as A. oleivorans according to the GTDB. To our knowledge, this is the first report of A.oleivorans as a human pathogen. A 27-year-old patient experienced A. oleivorans bacteremia at the outset of treatment. The patient had been diagnosed with VSAA and and had a history of residing in a harbor development zone. Approximately 10 days before admission, the patient developed a fever, which was further complicated by respiratory tract and lower extremity soft tissue infections. This MDR isolate was sensitive to cefoperazone-sulbactam, fluoroquinolones, and tetracycline and resistant to carbapenems (Supplementary Table 2). The patient was eventually cured with ceftazidime-avibactam and tigecycline. The virulence and drug resistance genes of the A. oleivorans strain are shown in Fig. 3. This study found that the A. oleivorans strain did not carry common oxacillinases, such as OXA-23, OXA-24/40, OXA-51, OXA-58, and OXA-143. Instead, it carried a unique oxacillinase, OXA-325, which has not been previously reported in A. oleivorans.

The vital virulence factors (A) and drug-resistance genes (B) of the remaining Acinetobacter strains isolated from the bloodstream. The text above represents the types of genes, and the text below represents the names of genes. Blue squares indicate gene positives, while white squares indicate gene negatives. OMP, outer membrane proteins; LPS, lipopolysaccharide; PD, phospholipase D; QS, quorum-sensing system; PNAG, beta-1-6-linked poly-N-acetyl glucosamine; PBPs, penicillin-binding proteins; FQs, fluoroquinolones; Ms, macrolides; TCs, tetracyclines; A, ambler class A of β-lactams antibiotics