Infection
CAR T cell therapies may lead to reactivation of childhood viral infections
Research led by the Department of Pathology at Stanford University, California, has found that chimeric antigen receptor T (CAR T) cell therapy can potentially result in a reactivation of human herpesvirus 6 (HHV-6) by CAR T cells in patients.
In a paper titled “Latent human herpesvirus 6 is reactivated in CAR T cells,” published in Nature, the team gathered previous data surrounding CAR T therapy studies to investigate the in vivo reactivation of HHV-6 by CAR T cells in patients receiving CAR T cell therapy. The study sought to characterize the phenomenon of HHV-6 reactivation, particularly in the context of CAR T cell treatment for B cell lymphoma or leukemia.
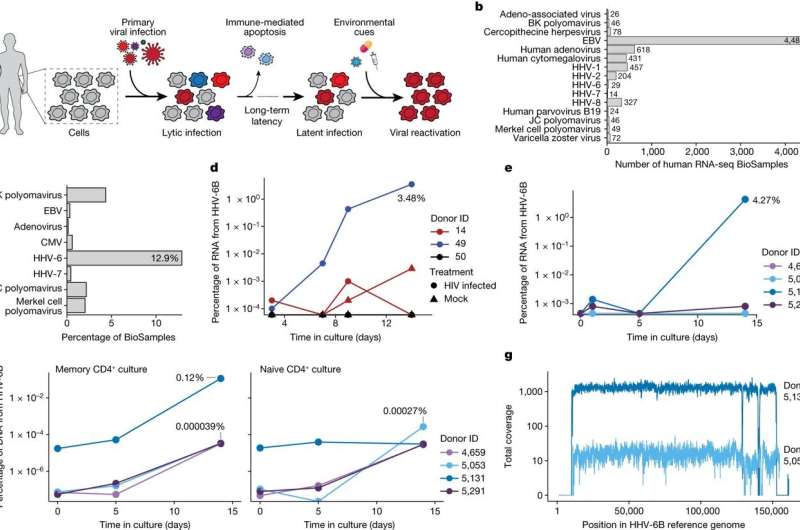
A reanalysis of scRNA-seq datasets from three cohorts of patients receiving autologous CAR T cell products was conducted. While no HHV-6 viral transcripts were detected in pre-infusion products, a significant increase in HHV-6+ cells was observed in post-infusion samples.
The study identified 28 cells expressing HHV-6B transcripts in post-infusion samples, with 13 resembling rare “super-expressor” cells among research-grade allogeneic CAR T cells. Detailed time course analysis of two patients with super-expressors revealed the presence of HHV-6+ cells a week into treatment, coinciding with clinical symptoms of immune effector-associated neurotoxicity syndrome. The symptoms, such as delirium and neurocognitive decline, align with HHV-6 viral presence in blood, though it may not be causal for all patients.
These findings suggest a link between T cell activation, proliferation, and culture duration with HHV-6 reactivation, emphasizing the need for further consideration in developing and monitoring CAR T cell therapies.
The study also found incidents of HHV-6B reactivation in standard CD4+ T cell cultures, confirming the potential association between cell therapy products and lytic HHV-6 infection that had been reported in clinical trials of CAR T.
Human herpesvirus 6B is nearly universally acquired (approximately 70%) by age three and causes a common childhood illness called exanthema subitum or roseola infantum. By adulthood, approximately 95% of people have encountered the virus. The virus is present in multiple cell and tissue types during primary infection, and the viral DNA continues to survive post-infection in peripheral blood mononuclear cells.
The authors emphasize the utility of comprehensive genomics analyses in implicating cell therapy products as potential sources contributing to viral infections. The correlation between HHV-6 reactivation and CAR T treatments raises the possibility of other latent viral reactivations across various cell therapies and suggests more investigation is required.
More information:
Caleb A. Lareau et al, Latent human herpesvirus 6 is reactivated in CAR T cells, Nature (2023). DOI: 10.1038/s41586-023-06704-2
© 2023 Science X Network
Citation:
CAR T cell therapies may lead to reactivation of childhood viral infections (2023, November 14)
retrieved 14 November 2023
from https://medicalxpress.com/news/2023-11-car-cell-therapies-reactivation-childhood.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.