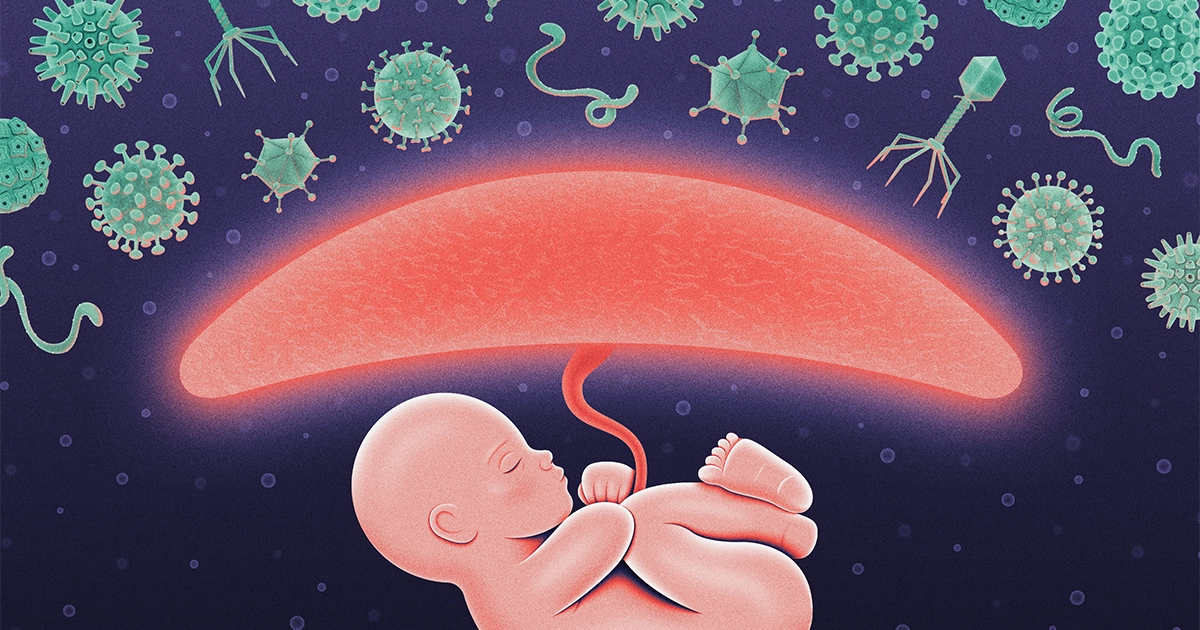
Infection
During Pregnancy, a Fake ‘Infection’ Protects the Fetus
Introduction
When you were a child, it seemed like an ingenious plan: Splash hot water on your face and stagger into the kitchen, letting out a moan that could make angels cry. One touch of your flushed forehead would convince your parents to diagnose a fever and keep you home from school.
No matter how elaborately planned and performed, these theatrics probably weren’t as persuasive as you had hoped. But new research, published this summer in Cell Host & Microbe, suggests that long before birth, a similar tactic helps developing humans and other mammals put on a more convincing show.
The study showed how the placenta — the embryonic organ that connects offspring and mother — uses a molecular trick to feign illness. By pretending it’s under viral attack, it keeps the immune system running at a gentle, steady pace to protect the enclosed fetus from viruses that slip past the mom’s immune defenses.
The discovery suggests that prior to infection, some cells may be able to activate a subtle immune response that can provide moderate protection in delicate tissues.
The idea of cells activating immune defenses preemptively “very much violates one of the views that immunologists have,” said Jonathan Kagan, an immunobiologist at Boston Children’s Hospital and Harvard Medical School who was not involved in the new study.
Because antiviral immune weapons can destroy tissues, cells typically turn them on only when there’s an active threat like an infection, Kagan said. Then, once the infection clears, those weapons are turned off as quickly as possible.
But the placenta breaks these rules, according to the new research. Somehow, it turns on defenses before they are necessary and then leaves them on without harming itself or the fetus.
“It protects but doesn’t damage,” said Hana Totary-Jain, an associate professor of molecular pharmacology at the University of South Florida in Tampa and lead author on the new paper. “Evolution is so smart.”
The Placenta Fakes Sick
Totary-Jain discovered the placenta’s sleight of hand by accident. She and her lab were researching a mega-cluster of genes — “a monster,” she said — that was expressed in the placenta. She was surprised to see that, in addition to activating genes that guide placental development, the mega-cluster had turned on the gene for interferon lambda, an immune signaling protein. Why was it active in healthy, uninfected cells?
It took years for Totary-Jain and her team to zero in on an answer: The placental cells had crafted a viral look-alike, using RNA harvested from their own genomes, to dupe their immune sensors.
Our genomes are molecular museums of evolutionary history. Since the beginning of life on Earth, viruses have inserted portions of their genetic material into their hosts’ DNA. Tucked between genes that code for proteins are genomic relics from ancient microbial invasions.
Introduction
One of the most common viral elements that persist in human genomes is a chunk of DNA called an Alu repeat. Alus constitute at least 13% of the human genome; there were over 300 copies in Totary-Jain’s mega-cluster. She suspected that those Alu repeats were turning on the immune system in the placenta. But her colleagues cautioned her against going down that road.
“The advice I was given was: ‘Don’t touch Alus, don’t work with Alus, forget about Alus,’” Totary-Jain said. The multitude of Alus in the genome makes it tough to unpack what a specific set may be doing.
But the data implicating Alus was too compelling to ignore. After years of careful experiments, Totary-Jain’s team showed that in the placenta, transcripts of Alu repeats formed snippets of double-stranded RNA — a molecular silhouette our cells recognize as viral in origin. Sensing the fake virus, the cell responded by producing interferon lambda.
“The cell is effectively dressing up as an infectious agent,” Kagan said. “The result is that it convinces itself that it’s infected, and then operates as such.”
Simmering Immunity
Immune responses can be destructive, and antiviral responses especially so. Because viruses are at their most dangerous when they’re already inside a cell, most immune strategies that target viral infections work in part by damaging and killing infected cells.
For that reason, cells cry “Virus!” at their own risk. In most tissues, Alu sequences are highly suppressed so that they never get a chance to mimic a viral attack. And yet that is the exact scenario the placenta seems to create on purpose. How does it balance the health of the growing embryo with a potentially risky immune response?
In experiments with mice, Totary-Jain’s team found that the placenta’s double-stranded RNAs and ensuing immune response didn’t seem to hurt the developing embryos. Instead they protected the embryos from Zika virus infection. The placental cells were able to toe the line — conferring protection on the embryos without cuing a self-destructive immune response — because they called in the gentler defenses of interferon lambda.
Typically the first responders to double-stranded Alu RNA escapees are type I and type II interferons, which quickly recruit destructive immune cells to the site of an infection, leading to tissue damage and even autoimmune disease. Interferon lambda, on the other hand, is a type III interferon. It acts locally by communicating only with cells within the tissue, generating a milder immune response — one that can be sustained long term in the placenta.
How placental cells manage to activate only interferon lambda, keeping the immune response simmering but never boiling over, is still a mystery. But Totary-Jain has an idea about why placental cells evolved this trick that other cells seemingly avoid: Since the placenta is discarded at birth, perhaps it can afford to take immune risks that other tissues can’t.
The findings reveal a new strategy the placenta has for protecting the fetus, apart from mom’s immune system. Since the mother’s immune response is dampened during pregnancy to prevent attacks on the genetically distinct embryonic cells, the placenta has had to develop extra defenses for the growing baby it supports.
However, this trick — a low-level immune response generated by a fake virus — may not be limited to the placenta. Researchers from Columbia University recently described a similar phenomenon in neurons. They observed RNAs from different genomic elements bound together in double strands to produce an immune response. In this instance, the immune system called in a more destructive type I interferon, but it was produced at low levels. The authors surmised that chronic low-level inflammation in the brain may keep infections under control, preventing major inflammation and neuronal death.
It’s possible, then, that this kind of immune trickery is more common than anyone thought. By studying how the immune system seems to break its own rules, scientists can better define what the rules are in the first place.
Quanta is conducting a series of surveys to better serve our audience. Take our biology reader survey and you will be entered to win free Quanta merchandise.