Congenital disorders
Preterm infant with a giant fetal neck lymphangioma
Introduction
Lymphatic malformations (LMs) are rare entities, sometimes difficult to treat. They can be life-threatening when intricately connected to airway structures. The International Society for the Study of Vascular Anomalies (ISSVA) classifies LMs as follows:
- Common (cystic) LM (with subtypes macrocystic, microcystic, mixed cystic)
- Generalized lymphatic anomaly (GLA)
- LM in Gorham–Stout disease
- Channel-type LM
- “Acquired” progressive lymphatic anomaly (so called acquired progressive “lymphangioma”)
- Primary lymphedema
and others.1 “Cystic hygroma” and “cystic lymphangioma” are historical terms for macrocystic LMs that typically arise in the neck.2 LMs are benign vascular malformations of the lymphatic system that most commonly present in the neck area.
LMs are congenital malformations of the fetal lymphatic system; that is, they are developmental defects in the connection between the lymphatic channels and the venous system or due to abnormal development of the lymphatic vessels. Nearly 75% occur primarily in the lymph gland–rich head and neck region; they can also occur in the body, limbs, armpits, retroperitoneum, mediastinum, and other areas.3,4 It is reported that the incidence of LMs in fetuses from 11 to 14 weeks of gestation is 0.6%, with 1 case per 6000 to 16,000 live births. The incidence of spontaneous abortion in such fetuses is approximately 1 in 750.5–7
These conditions can occur alone or in association with other fetal structural anomalies.8 Fetal malformation or nuchal thickening, which usually occurs between 11 and 23 weeks of gestation, is frequently associated with chromosomal abnormalities and warrants chromosomal analysis. Turner’s syndrome is the most common abnormality of this kind.9
The advancement of prenatal diagnostic technology has greatly increased the detection of fetal LMs. Once such a condition is identified, multidisciplinary consultation and counseling can help to clarify the specific concerns and perinatal management. The morbidity and mortality associated with LMs stem from the complications of tumoral growth. The growth of a neck mass, for example, can lead to compression or even displacement of the esophagus and trachea, followed by premature delivery, pleural effusion and ascites, fetal edema, neonatal airway obstruction, and serious respiratory and feeding problems in the newborn.10 Therefore treatment decisions in cases of LMs are always complex, as they depend on the patient’s symptoms, the clinical presentation, size and location of the mass, and possibly functional complications.11
Herein, we report a case of giant fetal neck LM in a preterm infant and the successful application of sclerotherapy. The treatment procedure followed ethical principles; all data were collected from chart reviews, and approval was obtained from our hospital’s institutional review board.
Case Presentation
A 31-year-old woman, gravida 1 para 0, conceived spontaneously. Ultrasound examination at 12 weeks of gestation showed a single fetus. The mother had a history of allergy to sulfa antibiotics; however, she and her partner reported no history of reactions to medications or substance abuse and no family history of congenital anomalies. In the first trimester, cell-free fetal DNA screening was performed and the result showed no obvious abnormalities. At 23+ weeks of gestation, the size of 7.2×6.5×6.3 cm septate cystic mass was found on the right side of the neck of the fetus by ultrasonography, which was considered to be LM of the fetal neck (Figure 1A). Fetal amniocentesis chromosome microarray analysis showed no significant abnormalities. Consultations and evaluations were conducted among the multidisciplinary team (comprising an obstetrician, pediatric surgeons, otorhinolaryngologists, interventional radiologists, neonatologists, pediatric cardiologists, anesthesiologists, and clinical geneticists).
 |
Figure 1 (A) Ultrasonography image of the fetal neck lymphatic malformation at 23+ weeks. (B) Ultrasonography images of the fetal neck lymphatic malformation at 27 weeks. C. Ultrasonography images of the fetal neck lymphatic malformation at 31 weeks and 5 days. |
During gestation, the septate cystic mass gradually increased in size, with no fetal hydrops (Figure 1B and C). At 29 weeks, Magnetic resonance imaging (MRI) of fetal neck was performed. The results revealed an irregular septate cystic mass on the right side of the fetal neck extending from the right auricle into the entrance of the thoracic entrance plane, with 6.5×6.3×7.4 cm in size. A patchy hemorrhage signal—1.9×2.9 cm in size—appeared in the posteroinferior cyst. The nasopharyngeal, oropharyngeal, and laryngopharyngeal cavities were obviously narrowed. The cervical trachea was obviously compressed and displaced. The diameter of the narrowest cervical tracheal lumen seen anteroposteriorly was 1.3 mm (Figure 2).
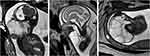 |
Figure 2 Magnetic resonance images of the fetal neck lymphatic malformation at 29 weeks. |
The mother and her partner chose to continue the pregnancy after extensively counseled by the multidisciplinary team. At 31 weeks and 2 days, the mother was admitted to our hospital, having noticed a decline in fetal movement for 2 days. Doppler flow imaging of the ductus venosus showed a reverse a-wave. Ultrasonography revealed a septated cystic mass 9.5×8.8×6.3 cm in size with a large cystic space of 3.3×2.3 cm, along with polyhydramnios, as the amniotic fluid index was 28.7 cm and the amniotic fluid volume 8.9 cm. The multidisciplinary team conducted comprehensive monitoring of the mother’s and fetus’s status. Because prolonged deceleration on electronic monitoring of the fetal heart suggested fetal distress, an emergency cesarean section was performed at 31 weeks and 5 days. A male baby weighing 1720 g was delivered with ex utero intrapartum treatment (EXIT) while maintaining the fetal placental circulation. The umbilical cord was extremely twisted in 53 laps, and there was a cystic mass (10×16×8 cm) on the right side of his neck (Figure 3A). He was then transferred to the neonatal intensive care unit under endotracheal intubation combined with positive pressure ventilation.
 |
Figure 3 (A) Image of the neonatal neck lymphatic malformation at birth. (B) Image of the neonatal neck lymphatic malformation 3 days after birth. (C) Image of the neck lymphatic malformation one year after birth. |
On the same day, ultrasound examination of the right neck revealed a septate cystic space 12.1×8.8×10.5 cm in size. On the next day, computed tomography (CT) revealed a huge irregular cystic mass of uneven density; the upper part of the mass was adjacent to the right temporal region and the lower part reached the right thoracic entrance plane, partially entering the thoracic cavity, which compressed the local esophagus and trachea to the left. Its CT value was approximately 18 HU. (Figure 4).
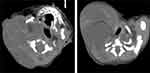 |
Figure 4 Computed tomography images of the neonatal neck lymphatic malformation 1 day after birth. |
At 3 days of age, due to progressive growth of the mass secondary to intralesional bleeding (Figure 3B), the neonate was treated by cervical sclerotherapy with bleomycin 1500 U. This intervention was uneventful. Thereafter, the cystic mass was found to be gradually shrinking in size, and extubation was performed 19 days later. Because the esophagus and trachea were still compressed to the left and the pharyngeal cavity and tracheal lumen remained narrowed, sclerotherapy was again performed with bleomycin 1000 U when the neonate was 1 month and 8 days of age, again uneventfully. At this point the mass was approximately 8×5×8 cm in size. The infant had no obvious symptoms of airway compression and no obvious dyspnea, hoarseness, or dysphagia. The infant was discharged 8 days after the second sclerotherapy and thereafter received regular follow-up visits. One year after his birth, the mass had disappeared (Figure 3C). He remained in good health after 2 1/2 years of follow-up.
Discussion
Fetal tumors are rare congenital anomalies, with an estimated incidence of 1/12,000 to 1/30,000 births.12 As one type of fetal neck tumor, fetal neck lymphatic malformation represents approximately 42% to 52% of all occurrences.13 The overall incidence rate is 1/1000 to 1/6000.14 Lymphatic malformation is the result of aberrant development of the lymphatic system, a process that is complete by the end of the first 2 months of gestation. These tumors are thought to arise from failure of the lymphatics to connect to the venous system. They can be also result from abnormal budding of the lymphatic tissue or from the persistence of isolated lymphatic rests, which retain their embryonic potential, become canalized and continue to secret lymph, thus generating cystic structures.15 They are considered to be benign malformations of the lymphatic system with no tendency toward malignant growth.16 Characteristic is a protein-rich fluid found in the nuchal region, behind and around the fetal neck; this region can extend the entire length of the fetus and show septations; it can also be observed in other tissues such as the limbs, body, mediastinum, and chest.8
Fetuses with LM are at high risk for adverse outcomes. The disease is frequently associated with chromosomal abnormalities, Turner’s syndrome being the most common. Genetic syndromes are also reported in LM. The most common are Noonan syndrome, multiple-pterygium syndrome, Fryns syndrome, and Neu–Laxova syndrome.17 In addition, data regarding 410 cases of first-trimester septated cystic hygroma from among 110,000 pregnancies in Ireland have confirmed associated abnormal chromosomal findings among such fetuses and an increased likelihood of structural anomalies among euploid fetuses with first-trimester septated cystic hygroma.18 Therefore, when fetal structural abnormalities are detected by ultrasound in the first trimester, an invasive prenatal diagnosis should be performed.
Given the particularity of the lesion in the fetal neck, the progressive progress of the lesion can cause compression symptoms and severe fetal intrauterine complications such as respiratory tract obstruction, nerve compression, and capsular hemorrhage. Newborns may have dyspnea, feeding difficulties, and other problems.10 Nearly 90% of such fetuses die before birth, 2% die in the neonatal period or will maintain an abnormal karyotype and/or other malformations. The mortality rate in the first year is around 12%. Only 8% of the survivors will be in good health.15,19,20
According to the size of the fluid-filled spaces in LM, they are classified as macrocystic (>2 cm), microcystic (<2 cm), or mixed.1,11,21 Owing to the availability of advanced imaging, the established LM staging system now plays an important role in evaluating the effects of treatment. The current de Serres staging system is as follows: stage I, unilateral infrahyoid; stage II, unilateral suprahyoid; stage III, unilateral suprahyoid and infrahyoid; stage IV, bilateral suprahyoid; and stage V, bilateral suprahyoid and infrahyoid.22–24
Prenatal ultrasound is the primary tool for the screening of lymphatic malformations; fetal MRI is a valuable complementary method to view enhanced global imaging of the mass and to visualize the fluid-filled airway and esophagus. It is also useful for in estimating the severity of compressive obstruction, as it can assesses the degree of tracheal compression before birth and evaluate the feasibility of postnatal endotracheal intubation. This is important for subsequent clinical decision making and treatment.25,26 Once the diagnosis has been confirmed, a multidisciplinary team approach is essential. To rule out associated congenital syndromes and aneuploidies, ancillary investigations such as amniocentesis with detailed karyotyping are also of paramount importance. Fetal echocardiography is recommended to screen the fetus for structural cardiac abnormalities and evaluating cardiac function.27,28
The most important part of the antenatal management of fetal neck tumors is assessing the degree of fetal airway obstruction. Lazar et al29 proposed a tracheoesophageal displacement index (TEDI) to do this. The TEDI was defined on fetal MRI as the sum of the lateral and ventral displacements (in millimeters) of the tracheoesophageal complex from its normal anatomic location at the ventral aspect of the cervical spine. A TEDI greater than 12 correlates strongly with a complicated airway (area under the curve = 0.80).
Another important aspect of the antenatal management of these cases is an assessment of amniotic fluid volume. An increase in the amniotic fluid index and the development of polyhydramnios indicate impaired fetal swallowing as a consequence of esophageal and tracheal displacement and compression.12 When there is severe polyhydramnios causing preterm contractions and dyspnea, amnioreduction may be required. This may decrease the risk of preterm labor, which has direct effects on neonatal mortality and morbidity.30
Considering the possibility of adverse perinatal outcomes caused by LM complications, early intervention is recommended, including timely termination of pregnancy, intrauterine treatment, and postpartum intervention. The decision between treatment and termination of pregnancy is usually based on the presence of abnormal karyotypes or severe structural abnormalities, which require systematic monitoring of disease progression and any related complications. In early reports, for cases of spontaneous regression of CH before 20 weeks, with normal chromosomal karyotypes and ultrasound examinations, continued pregnancy may be considered. In cases of persistent CH after 20 weeks, with a normal karyotype but obvious morphologic abnormalities, termination of the pregnancy is advocated.19
Until now, therapeutic options for severe lymphatic malformations were limited to surgical and/or ablative techniques or sclerotherapy. Furthermore, some authors have published studies on in-utero therapy with drugs for fetal lymphatic malformations. Mikovic et al30 first evaluated prenatal sclerosing therapy of fetal neck lymphangiomas with OK-432; based on 2 cases, the results were positive. Livingston et al31 reported the first successful case of in-utero therapy with maternal oral rapamycin for a rapidly enlarging obstructive fetal cervical LM. They found fetal therapy with rapamycin to be safe and effective in managing this severe malformation.
In cases of fetal neck masses with suspicion of airway obstruction, a caesarean section with the EXIT procedure should be scheduled. Effective uteroplacental blood flow should be maintained during the EXIT procedure, with the fetus being intubated immediately after partial delivery from the uterus to ensure patency of the airway.12,27 In this study, MRI of the fetal neck showed obvious compression, displacement, and stenosis of the cervical trachea, with the narrowest diameter being 1.3 mm. Moreover, there were symptoms of polyhydramnios, indicating obvious compression of the fetal airway. Therefore we performed an EXIT procedure.
Most lymphatic malformations persist postnatally, with spontaneous regression rates between 2.3% and 41%.32 Postnatal treatment depends mainly on the symptoms, clinical manifestations, size and location of the cyst, and possible functional complications; thus and a consensus with the patient’s family must be reached.
Historically, surgical resection of a neck mass was the standard therapeutic choice. However, the associated lymphatic malformation can infiltrate the oropharynx, involving surrounding structures such as the cranial nerve and main blood vessels, making it difficult to achieve complete resection. The postoperative recurrence rate in such cases—related complications such as fistulization, infection, dehiscence, and cosmetic problems—eventually led to the development of alternative approaches.11
More recently, sclerotherapy has provided an alternative to surgery, mainly in isolated, nonenlarging macrocystic LMs and widespread invasiveness.33 This technique is accurate, minimally invasive, and safe; it also has a lower recurrence rate than that following surgery.23 It can also be used in conjunction with surgical resection when the initial treatment fails. The sclerosants available for the sclerotherapy include bleomycin, OK-432, tetracycline, cyclophosphamide, doxycycline, lauromacrogol, ethanol and others, which act by causing irritation of the endothelial lining of the lymphangioma, leading to inflammation, fibrosis, and involution.28 Bleomycin, as one of the commonly used sclerosants, was first used to treat LMs by intralesional injection in 1977, its mainly through endothelial injury and induce inflammatory reaction, resulting in thickening and occlusion of the cyst wall, which leads to the cyst cavity shrinking and even healing. Compared with other sclerosing agents, one major apparent advantage of bleomycin is the relatively minimal inflammatory reaction and edema post injection, which is relatively friendly in treating sensitive areas, such as the airway, orbit, mouth, where edema cannot be tolerated.34,35 Niramis et al36 also report that in a large series of 70 patients treated with intralesional bleomycin injection, 33 (47.1%) had complete clinical remission, 25 (35.8%) had good remission, and only 12 (17.1%) patients failed to respond. The main complications of intralesional bleomycin application include erythema, ulceration, hyperpigmentation, scarring, and the systemic toxicity, pulmonary fibrosis and renal insufficiency that may be caused by increased dose of bleomycin are also the concerns of our treatment. It has been reported that when bleomycin is measured up to 300 mg, the probability of pulmonary toxicity is 8.5%.37 Therefore, it is also important to monitor lung CT and lung function tests during long-term follow-up. In this case, the lymphatic malformation was located in the neck and the mass was up to 12.1×8.8 × 10.5 cm in size. Considering these combined factors, bleomycin was selected as sclerosing agents in therapy. At subsequent telephone follow-up, the patient’s lung CT and lung function show good health.
Medical therapy has been considered for stable, nongrowing LMs not involving the deep structures in spontaneously breathing patients. It comprised the oral administration of rapamycin, an mTOR inhibitor whose antiangiogenic activity impairs the function of the vascular endothelial growth factor.11,38 Strychowsky et al39 used sirolimus as primary treatment in 19 patients with refractory cervicofacial lymphatic malformations and found that this approach was often efficacious, with few adverse events.
In this case, we performed intralesional sclerotherapy with the injection of bleomycin and had a favorable outcome.
Conclusion
In conclusion, treatment decisions in cases of LM depend on the patient’s symptoms, the clinical presentation, the size and location of the cyst, and possible functional complications. Accurate prenatal diagnosis, well-trained multidisciplinary teams, and perinatal imaging support help improve maternal perinatal outcomes and fetal and neonatal morbidity and mortality. Our experience with the intralesional injection of bleomycin in this case also proved favorable, but a multidisciplinary long-term follow-up is still needed.
Data Sharing Management
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Ethics Approval and Consent to Participate
This study was approved by the ethics committees at the West China Second University Hospital of Sichuan University (2022-221).
Informed Consent
Written informed consent for publication of this case report and any accompanying images was obtained from the parents of the patients.
Acknowledgments
We feel grateful for the doctors and staff who have been involved in this work.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by the Natural Science Foundation of Sichuan (2022NSFSC0659) and the Science Foundation of Chengdu (No.2021-YF05-01532-SN).
Disclosure
The authors report no conflicts of interest associated with this work.
References
1. Wassef M, Blei F, Adams D, et al. Vascular anomalies classification: recommendations from the International Society for the Study of Vascular Anomalies. Pediatrics. 2015;136(1):e203–e214. doi:10.1542/peds.2014-3673
2. Bagrodia N, Defnet AM, Kandel JJ. Management of lymphatic malformations in children. Curr Opin Pediatr. 2015;27(3):356–363. doi:10.1097/MOP.0000000000000209
3. Chen HY, Zheng JQ, Zhang HP. A case report of Turner syndrome associated with fetal nuchal cystic hygroma and bilateral syndactyly of the hands and feet. Ital J Pediatr. 2019;45(1):85. doi:10.1186/s13052-019-0680-4
4. Kulungowski AM, Patel M. Lymphatic Malformations. Semin Pediatr Surg. 2020;29(5):150971. doi:10.1016/j.sempedsurg.2020.150971
5. Sannoh S, Quezada E, Merer DM, Moscatello A, Golombek SG. Cystic hygroma and potential airway obstruction in a newborn: a case report and review of the literature. Cases J. 2009;2(1):48. doi:10.1186/1757-1626-2-48
6. Noia G, Maltese PE, Zampino G, et al. Cystic Hygroma: a Preliminary Genetic Study and a Short Review from the Literature. Lymphat Res Biol. 2019;17(1):30–39. doi:10.1089/lrb.2017.0084
7. Dubois J, Thomas-Chaussé F, Soulez G. Common (Cystic) Lymphatic Malformations: current Knowledge and Management. Tech Vasc Interv Radiol. 2019;22(4):100631. doi:10.1016/j.tvir.2019.100631
8. Mastromoro G, Guadagnolo D, Khaleghi Hashemian N, et al. A Pain in the Neck: lessons Learnt from Genetic Testing in Fetuses Detected with Nuchal Fluid Collections, Increased Nuchal Translucency versus Cystic Hygroma-Systematic Review of the Literature, Meta-Analysis and Case Series. Diagnostics. 2022;13(1):48. doi:10.3390/diagnostics13010048
9. Ganapathy R, Guven M, Sethna F, Vivekananda U, Thilaganathan B. Natural history and outcome of prenatally diagnosed cystic hygroma. Prenat Diagn. 2004;24(12):965–968. doi:10.1002/pd.991
10. Jiao-Ling L, Hai-Ying W, Wei Z, Jin-Rong L, Kun-Shan C, Qian F. Treatment and prognosis of fetal lymphangioma. Eur J Obstet Gynecol Reprod Biol. 2018;231:274–279. doi:10.1016/j.ejogrb.2018.10.031
11. Gaffuri M, Torretta S, Iofrida E, et al. Multidisciplinary management of congenital giant head and neck masses: our experience and review of the literature. J Pediatr Surg. 2019;54(4):733–739. doi:10.1016/j.jpedsurg.2018.09.018
12. Kornacki J, Skrzypczak J. Neck tumors-antenatal and intrapartum management. Ginekol Pol. 2017;88(5):266–269. doi:10.5603/GP.a2017.0050
13. Howarth ES, Draper ES, Budd JL, Konje JC, Clarke M. Population-based study of the outcome following the prenatal diagnosis of cystic hygroma. Prenat Diagn. 2005;25(4):286–291. doi:10.1002/pd.1100
14. Fisher R, Partington A, Dykes E. Cystic hygroma: comparison between prenatal and postnatal diagnosis. J Pediatr Surg. 1996;31(4):473–476. doi:10.1016/s0022-3468(96)90477-7
15. Tica OS, Gug C, Tica AA, et al. A unique case of recurrent fetal cystic hygroma: first fetus with an inherited heteromorphism of chromosome 1 (1qh+) and the second fetus with 69XXX triploidy. Rom J Morphol Embryol. 2020;61(3):935–940. doi:10.47162/RJME.61.3.34
16. Perkins JA, Manning SC, Tempero RM, Cunningham MJ. Lymphatic malformations: current cellular and clinical investigations. Otolaryngol Head Neck Surg. 2010;142(6):789–794. doi:10.1016/j.otohns.2010.02.025
17. Pan M, Liu YN, Xu LL, Li D-Z. First-trimester cystic hygroma and neurodevelopmental disorders: the association to remember. Taiwan J Obstet Gynecol. 2020;59(6):960–962. doi:10.1016/j.tjog.2020.09.029
18. Malone CM, Mullers S, Kelliher N, et al. Euploid First-Trimester Cystic Hygroma: a More Benign Entity than Previously Thought? Fetal Diagn Ther. 2021;48(9):667–671. doi:10.1159/000519056
19. Gedikbasi A, Gul A, Sargin A, Ceylan Y. Cystic hygroma and lymphangioma: associated findings, perinatal outcome and prognostic factors in live-born infants. Arch Gynecol Obstet. 2007;276(5):491–498. doi:10.1007/s00404-007-0364-y
20. Descamps P, Jourdain O, Paillet C, et al. Etiology, prognosis and management of nuchal cystic hygroma: 25 new cases and literature review. Eur J Obstet Gynecol Reprod Biol. 1997;71(1):3–10. doi:10.1016/s0301-2115(96)02590-0
21. Benazzou S, Boulaadas M, Essakalli L. Giant pediatric cervicofacial lymphatic malformations. J Craniofac Surg. 2013;24(4):1307–1309. doi:10.1097/SCS.0b013e3182942b8f
22. Adams MT, Saltzman B, Perkins JA. Head and neck lymphatic malformation treatment: a systematic review. Otolaryngol Head Neck Surg. 2012;147(4):627–639. doi:10.1177/0194599812453552
23. Aluffi Valletti P, Brucoli M, Boffano P, et al. A single-center experience in the management of head and neck lymphangiomas. Oral Maxillofac Surg. 2020;24(1):109–115. doi:10.1007/s10006-020-00832-z
24. de Serres LM, Sie KC, Richardson MA. Lymphatic malformations of the head and neck. A proposal for staging. Arch Otolaryngol Head Neck Surg. 1995;121(5):577–582. doi:10.1001/archotol.1995.01890050065012
25. Kang Y, Ma Y, Jiang X, Lin X, Zhao F. Fetal giant right cervical cyst causing severe tracheal compression: a case report. Medicine. 2019;98(31):e16670. doi:10.1097/MD.0000000000016670
26. Ng TW, Xi Y, Schindel D, Beavers A, Santiago-Munoz P, Bailey AA. Fetal Head and Neck Masses: MRI Prediction of Significant Morbidity. AJR Am J Roentgenol. 2019;212(1):215–221. doi:10.2214/AJR.18.19753
27. Olutoye OO, Olutoye OA. EXIT procedure for fetal neck masses. Curr Opin Pediatr. 2012;24(3):386–393. doi:10.1097/MOP.0b013e3283531b51
28. Behera S, Bawa M, Kanojia RP, Saha PK, Singh T, Samujh R. Outcome of antenatally diagnosed cystic hygroma-Lessons learnt. Int J Pediatr Otorhinolaryngol. 2020;138:110227. doi:10.1016/j.ijporl.2020.110227
29. Lazar DA, Cassady CI, Olutoye OO, et al. Tracheoesophageal displacement index and predictors of airway obstruction for fetuses with neck masses. J Pediatr Surg. 2012;47(1):46–50. doi:10.1016/j.jpedsurg.2011.10.022
30. Mikovic Z, Simic R, Egic A, et al. Intrauterine treatment of large fetal neck lymphangioma with OK-432. Fetal Diagn Ther. 2009;26(2):102–106. doi:10.1159/000238111
31. Livingston J, Alrowaily N, John P, et al. Fetal therapy using rapamycin for a rapidly enlarging, obstructive, cervical lymphatic malformation: a case report. Prenat Diagn. 2021;41(7):884–887. doi:10.1002/pd.5925
32. Kalwani NM, Rockson SG. Management of Lymphatic Vascular Malformations: a Systematic Review of the Literature. J Vasc Surg Venous Lymphat Disord. 2021;9(4):1077–1082. doi:10.1016/j.jvsv.2021.01.013
33. Tu JH, Do HM, Patel V, Yeom KW, Teng JMC. Sclerotherapy for lymphatic malformations of the head and neck in the pediatric population. J Neurointerv Surg. 2017;9(10):1023–1026. doi:10.1136/neurintsurg-2016-012660
34. Yılmaz H, Yılmaz Ö, Çamlıdağ İ, Belet Ü, Akan H. Single center experience with intralesional bleomycin sclerotherapy for lymphatic malformations. Jpn J Radiol. 2017;35(10):590–596. doi:10.1007/s11604-017-0672-5
35. Perkins JA, Manning SC, Tempero RM, et al. Lymphatic malformations: review of current treatment. Otolaryngol Head Neck Surg. 2010;142(6):795–803, 803.e1. doi:10.1016/j.otohns.2010.02.026
36. Niramis R, Watanatittan S, Rattanasuwan T. Treatment of cystic hygroma by intralesional bleomycin injection: experience in 70 patients. Eur J Pediatr Surg. 2010;20(3):178–182. doi:10.1055/s-0030-1247548
37. O’Sullivan JM, Huddart RA, Norman AR, Nicholls J, Dearnaley DP, Horwich A. Predicting the risk of bleomycin lung toxicity in patients with germ-cell tumours. Ann Oncol. 2003;14(1):91–96. doi:10.1093/annonc/mdg020
38. Amodeo I, Colnaghi M, Raffaeli G, et al. The use of sirolimus in the treatment of giant cystic lymphangioma: four case reports and update of medical therapy. Medicine. 2017;96(51):e8871. doi:10.1097/MD.0000000000008871
39. Strychowsky JE, Rahbar R, O’Hare MJ, Irace AL, Padua H, Trenor CC. Sirolimus as treatment for 19 patients with refractory cervicofacial lymphatic malformation. Laryngoscope. 2018;128(1):269–276. doi:10.1002/lary.26780
