Cardiovascular
Influenza vaccination and major cardiovascular risk: a systematic review and meta-analysis of clinical trials studies
Abstract
Cardiovascular events remain a substantial global health concern, necessitating innovative strategies for prevention. This study aims to assess the potential impact of influenza vaccination on major cardiovascular events. A search of the medical English literature was conducted using PubMed/MEDLINE, EMBASE, and the Cochrane CENTRAL up to 1 August 2023. Meta-analysis and stratified analyses were performed to investigate specific outcomes, including myocardial infarction (MI), cardiovascular death, and stroke. Pooled relative risks (RR) along with their 95% confidence intervals (CI) were calculated to evaluate the associations. A comprehensive analysis was conducted on a total of 9059 patients, with 4529 patients receiving the influenza vaccine and 4530 patients receiving a placebo. Among patients who received the influenza vaccine, a notable reduction in the occurrence of major cardiovascular events was observed, with 517 cases compared to 621 cases in the placebo group (RR 0.70; 95% CI 0.55–0.91). The stratified analysis revealed a decreased risk of MI in vaccinated patients (RR 0.74; 95% CI 0.56–0.97) and a significant reduction in cardiovascular death events (RR 0.67; 95% CI 0.45–0.98). This study provides compelling evidence that influenza vaccination is associated with a decreased risk of major cardiovascular events, particularly myocardial infarction, and cardiovascular death. These findings highlight the potential of influenza vaccination as an adjunctive strategy in cardiovascular disease prevention. Further research and exploration of underlying mechanisms are warranted to elucidate the observed beneficial effects.
Introduction
The significance of influenza vaccination has long been acknowledged in preventing seasonal flu infections, particularly in high-risk populations1,2,3. There are strong recommendations for influenza vaccination where six months and older people especially adults should be vaccinated against influenza4.
Cardiovascular diseases (CVDs) including hypertensive heart disease, ischemic heart disease, atrial fibrillation cerebrovascular disease like stroke, endocarditis, peripheral vascular disease, and other related cardiovascular diseases are a leading cause of morbidity and mortality worldwide5,6.
Differing viewpoints exist regarding the impact of influenza vaccination on CVDs. While certain observational investigations suggest a favorable correlation between influenza vaccination and the reduction in occurrences of cardiovascular incidents like acute myocardial infarction (MI), contrasting epidemiological studies propose the limited efficacy of influenza vaccines7,8,9,10.
Consequently, a comprehensive and updated study becomes imperative. This updated meta-analysis seeks to assess whether a connection exists between influenza vaccination and a decreased likelihood of experiencing cardiovascular events.
Methods
Search strategy
A search of the medical English literature was conducted using PubMed/MEDLINE, EMBASE, and the Cochrane CENTRAL up to 1 August 2023. A thorough review was conducted on randomized controlled trials (RCTs) exploring the potential link between influenza vaccination and the subsequent risk of developing CVDs.
The search terms were: myocardial disease and influenza vaccines (Table S1). This study adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement (PRISMA) for its design and reporting (Table S2) (Prospero ID: CRD42023450694)11.
Study selection
The records obtained from the database searches were amalgamated, and redundancy was addressed through employment of EndNote X7 (Thomson Reuters, Toronto, ON, Canada). Subsequently, two evaluators (MZ and MJN) carried out individual assessments of the records, considering both their title/abstract and full text, in order to exclude any entries that did not align with the study’s objectives.
The studies included in the analysis met the following criteria based on the PICOs:
Participants: The patients with a diagnosis of CVDs.
Intervention: Patients with CVDs who received influenza vaccine.
Comparison: Patients with CVDs who received a placebo.
Outcome: Lower risk of cardiovascular events.
Conference abstracts, case reports, and studies comparing high and low doses of influenza vaccination were excluded.
Data extraction
Data extraction was conducted by two investigators (MZ and MJN) and recorded in a Microsoft Excel spreadsheet (XP professional edition; Microsoft Corp, Redmond, WA). The extracted information encompassed various key elements, including the first author’s name, publication date, study type, definitions of cases and controls, the number of cases and controls, and the outcomes of the studies.
Quality assessment
The quality assessment of the included studies was conducted by two reviewers (MJN and MZ) using the Cochrane tool12. In case of any discrepancies, a third reviewer (FO) was involved. This instrument encompasses a range of domains, which encompass random sequence generation, allocation concealment, blinding of both participants and personnel, blinding of assessors for outcomes, completeness of outcome data, and additional considerations such as selective reporting and potential sources of bias. Each study was categorized as having a low risk of bias when no concerns regarding bias were identified, a high risk of bias when there were concerns about bias or an unclear risk of bias when there was insufficient information available.
Statistical analysis
The combined outcomes were presented as risk ratios along with corresponding 95% confidence intervals (CIs). To evaluate the level of variability among the studies, both the I2 value and p-value were employed. In cases where minimal statistical heterogeneity was observed (I2 ≤ 50% or p ≥ 0.1), the fixed-effect model was applied. Conversely, if substantial inter-study heterogeneity was identified (I2 > 50% or p < 0.1), the random-effects model was utilized. Assessment of between-study heterogeneity involved the application of Cochran's Q test and the I2 statistic. To assess potential publication bias, Begg's test was employed, where a significance level of p < 0.05 indicated noteworthy publication bias. All analytical procedures were conducted using Comprehensive Meta-Analysis Software, Version 3.0 (Biostat, Englewood, NJ).
Results
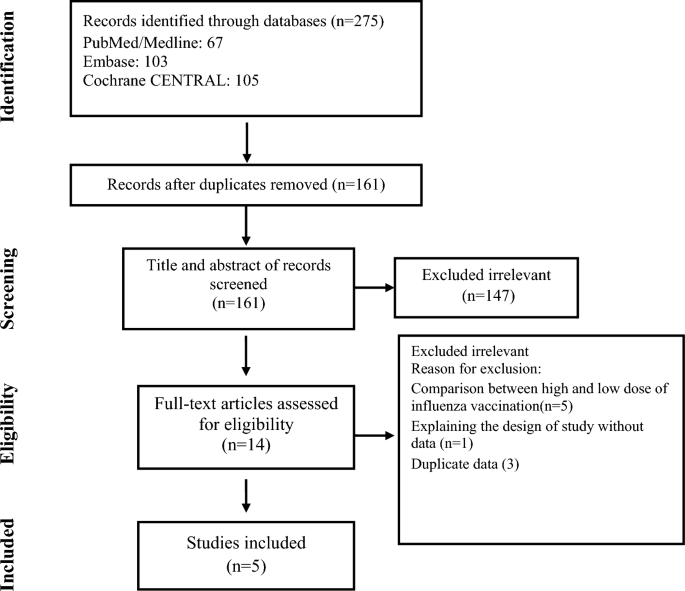
Out of the 275 articles initially identified, five studies comprising 9059 patients met the predetermined inclusion criteria, as illustrated in Fig. 18,13,14,15,16. The key characteristics of these selected studies are summarized in Table 1. Among the participants, a total of 4529 individuals were randomly assigned to receive a standard intramuscular influenza vaccination, while 4530 were assigned to receive an intramuscular placebo. The average age of the participants was 61.3 years, and they were followed up for an average duration of 9 months. The majority of the trials exhibited robust practices in terms of randomization, allocation concealment, and masking, aligning with the Cochrane criteria for high quality (low risk of bias), except for one trial (Table 2).
Flow chart of study selection for inclusion in the systematic review and meta-analysis.
Pooled major cardiovascular events
Within the cohort of 4529 patients who underwent influenza vaccination, a total of 517 individuals experienced significant cardiovascular events, in contrast to 621 cases among the 4530 patients who were administered a placebo. The calculated risk ratio (RR) was 0.70, indicating a noteworthy reduction in risk, accompanied by a 95% confidence interval (CI) of 0.55–0.91 (as depicted in Fig. 2).

Major cardiovascular events for influenza vaccine versus control.
Pooled stratified analysis
Stratified analyses are shown in Table 3. MI occurred in 86 of the 4529 patients who received influenza vaccine compared with 116 of the 4530 patients who received placebo (RR 0.74; 95% CI 0.56–0.97) (Fig. 3). The RR of developing cardiovascular death events after influenza vaccination was 0.67 (95% CI 0.45–0.98) (Fig. 4). Thus, influenza vaccination significantly decreased the risk of cardiovascular death (P-value = 0.04). There was not any significant association between influenza vaccination and risk of stroke (P-value = 0.7) (Fig. 5).

MI events for influenza vaccine versus control.

Stroke events for influenza vaccine versus control.

Cardiovascular death events for influenza vaccine versus control.
Discussion
Revealing a compelling insight into the potential benefits of influenza vaccination, our comprehensive meta-analysis, based on the latest randomized controlled trial (RCT) data, demonstrates a significant interaction between influenza vaccination and the reduction of major cardiovascular events. Notably, patients who received the influenza vaccine experienced a remarkable risk reduction of over 20% in cardiovascular death. These findings underscore the potential impact of influenza vaccination in safeguarding against adverse cardiovascular outcomes among vulnerable patient populations.
Mechanisms of the potential relationship
Understanding the potential mechanisms underlying this association is paramount to comprehending the clinical and public health implications of our findings17.
Inflammation Modulation Influenza infections are known to trigger systemic inflammation, and this inflammatory response may contribute to the progression of atherosclerosis and CVDs18,19,20.
Immune System Modulation Influenza vaccination activates the immune system, potentially influencing not only the response to influenza viruses but also the broader immune environment. A well-regulated immune system is essential for cardiovascular health, and the modulation of immune responses by vaccination may contribute to the observed cardiovascular benefits18,19,21.
Prevention of Secondary Infections Influenza infections can weaken the immune system, making individuals more susceptible to secondary infections22. Secondary infections, especially respiratory infections, can exacerbate cardiovascular conditions. Influenza vaccination’s role in preventing these secondary infections may indirectly contribute to the reduction in CVDs.
Stability of Atherosclerotic Plaques Influenza infections have been implicated in the destabilization of atherosclerotic plaques, leading to acute cardiovascular events23,24. Influenza vaccination may play a role in maintaining the stability of these plaques, reducing the likelihood of rupture and subsequent cardiovascular complications.
Comparisons with other studies
Our results align with the Behrouzi et al. meta-analysis published in 2022, which also demonstrated a decreased risk of CVDs in patients receiving influenza vaccination25. However, a more detailed analysis of the similarities and differences between our study and Behrouzi et al. is warranted.
In our study, we conducted stratified analyses based on MI, stroke, and cardiovascular death. This approach allowed us to focus specifically on a population with pre-existing CVDs, aiming to isolate and better understand the direct impact of influenza vaccination on individuals with established cardiovascular conditions. This differs from Behrouzi et al. as we deliberately excluded studies involving sample populations with specific comorbidities to ensure a more targeted examination. To delve deeper into these distinctions, it’s crucial to note that our study’s exclusion criteria were designed to minimize the influence of confounding factors. This deliberate choice to focus exclusively on a target population with pre-existing CVDs enhances the internal validity of our findings and provides a clearer picture of the direct relationship between influenza vaccination and cardiovascular outcomes. While our calculated RR for major cardiovascular events (0.70; 95% CI 0.55–0.91) was slightly higher than Behrouzi et al. (0.66; 95% CI 0.53–0.83), the consistency in direction and statistical significance reinforces the robustness of the observed association. This nuanced comparison underscores the importance of considering study design and inclusion criteria when interpreting the results of meta-analyses in the context of influenza vaccination and cardiovascular outcomes.
Implications of the findings
The observed interaction between influenza vaccination and reduced cardiovascular mortality among patients with recent CVDs is both clinically and epidemiologically significant. Cardiovascular diseases, including heart attacks and strokes, remain the leading causes of mortality worldwide. The potential of influenza vaccination to yield substantial reductions in cardiovascular mortality in this vulnerable patient group warrants serious attention from healthcare providers, policymakers, and researchers.
Clinical relevance and public health impact
By targeting patients with recent CVDs for influenza vaccination, healthcare providers have a potential opportunity to mitigate the risk of cardiovascular death in a cost-effective and widely available manner. Influenza vaccination programs could be tailored to prioritize this high-risk group, thus potentially reducing the overall burden on healthcare systems and improving patient outcomes.
Conclusion
The evidence from the most recent meta-analysis of RCT data is compelling, revealing a significant interaction between influenza vaccination and the reduction of major cardiovascular events among patients with recent CVDs. This finding underscores the potential benefits of targeting this high-risk group for vaccination. Further research is warranted to elucidate the precise mechanisms driving this association and to explore the long-term impact of influenza vaccination on cardiovascular outcomes. In the meantime, healthcare providers and policymakers should take heed of these findings and consider prioritizing influenza vaccination for patients with recent CVDs as a feasible and potentially life-saving preventive measure.
Data availability
The data used to support the findings of this study are included within the supplementary files.
References
-
Treanor, J. J. Influenza vaccination. N. Engl. J. Med. 375, 1261–1268 (2016).
Google Scholar
-
Azziz-Baumgartner, E., Grohskopf, L. & Patel, M. Realizing the potential of maternal influenza vaccination. Jama 325, 2257–2259 (2021).
Google Scholar
-
Wong, S.-S. & Webby, R. J. Traditional and new influenza vaccines. Clin. Microbiol. Rev. 26, 476–492 (2013).
Google Scholar
-
Fiore, A. E., Bridges, C. B. & Cox, N. J. Seasonal influenza vaccines. In Vaccines for Pandemic Influenza, 43–82 (2009).
-
Nabel, E. G. Cardiovascular disease. N. Engl. J. Med. 349, 60–72 (2003).
Google Scholar
-
Li, Y., Cao, G.-Y., Jing, W.-Z., Liu, J. & Liu, M. Global trends and regional differences in incidence and mortality of cardiovascular disease, 1990–2019: findings from 2019 global burden of disease study. Eur. J. Prev. Cardiol. 30, 276–286 (2023).
Google Scholar
-
Clar, C., Oseni, Z., Flowers, N., Keshtkar-Jahromi, M. & Rees, K. Influenza vaccines for preventing cardiovascular disease. Cochrane Database Syst. Rev. https://doi.org/10.1002/14651858.CD005050.pub3 (2015).
Google Scholar
-
Phrommintikul, A. et al. Influenza vaccination reduces cardiovascular events in patients with acute coronary syndrome. Eur. Heart J. 32, 1730–1735 (2011).
Google Scholar
-
Cheng, Y. et al. Effects of influenza vaccination on the risk of cardiovascular and respiratory diseases and all-cause mortality. Ageing Res. Rev. 62, 101124 (2020).
Google Scholar
-
Nichol, K. L. et al. Influenza vaccination and reduction in hospitalizations for cardiac disease and stroke among the elderly. N. Engl. J. Med. 348, 1322–1332 (2003).
Google Scholar
-
Moher, D., Liberati, A., Tetzlaff, J., Altman, D. G., PRISMA Group*. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 151, 264–269 (2009).
Google Scholar
-
Higgins, J. P. et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. Risk assessment tool. Bmj 343, d5928 (2011).
-
Loeb, M. et al. Influenza vaccine to reduce adverse vascular events in patients with heart failure: A multinational randomised, double-blind, placebo-controlled trial. Lancet Glob. Health 10, e1835–e1844 (2022).
Google Scholar
-
Fröbert, O. et al. Influenza vaccination after myocardial infarction: A randomized, double-blind, placebo-controlled, multicenter trial. Circulation 144, 1476–1484 (2021).
Google Scholar
-
Ciszewski, A. et al. Influenza vaccination in secondary prevention from coronary ischaemic events in coronary artery disease: FLUCAD study. Eur. Heart J. 29, 1350–1358 (2008).
Google Scholar
-
Gurfinkel, E. P., de la Fuente, R. L., Mendiz, O. & Mautner, B. Influenza vaccine pilot study in acute coronary syndromes and planned percutaneous coronary interventions: The FLU Vaccination Acute Coronary Syndromes (FLUVACS) study. Circulation 105, 2143–2147 (2002).
Google Scholar
-
Maniar, Y. M., Al-Abdouh, A. & Michos, E. D. Influenza vaccination for cardiovascular prevention: further insights from the IAMI trial and an updated meta-analysis. Curr. Cardiol. Rep. 24, 1327–1335 (2022).
Google Scholar
-
Aidoud, A. et al. Influenza vaccination as a novel means of preventing coronary heart disease: Effectiveness in older adults. Vaccine 38, 4944–4955 (2020).
Google Scholar
-
Ciszewski, A. Cardioprotective effect of influenza and pneumococcal vaccination in patients with cardiovascular diseases. Vaccine 36, 202–206 (2018).
Google Scholar
-
Zangiabadian, M. et al. Protective effect of influenza vaccination on cardiovascular diseases: A systematic review and meta-analysis. Sci. Rep. 10, 20656 (2020).
Google Scholar
-
Gopal, R., Marinelli, M. A. & Alcorn, J. F. Immune mechanisms in cardiovascular diseases associated with viral infection. Front. Immunol. 11, 570681 (2020).
Google Scholar
-
Christopoulou, I., Roose, K., Ibañez, L. I. & Saelens, X. Influenza vaccines to control influenza-associated bacterial infection: Where do we stand?. Expert Rev. Vaccines 14, 55–67 (2015).
Google Scholar
-
Skaarup, K. G., Modin, D., Nielsen, L., Jensen, J. U. S. & Biering-Sørensen, T. Influenza and cardiovascular disease pathophysiology: Strings attached. Eur. Heart J. Suppl. 25, A5–A11 (2023).
Google Scholar
-
Brennan, A. C., Campbell, R. T. & Lee, M. M. Vol. 25 1693–1695 (Wiley Online Library, 2023).
-
Behrouzi, B. et al. Association of influenza vaccination with cardiovascular risk: A meta-analysis. JAMA Netw. Open 5, e228873–e228873 (2022).
Google Scholar
Author information
Authors and Affiliations
Contributions
All the authors contributed equally and reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary Table S1.
Supplementary Table S2.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
Reprints and Permissions
About this article
Cite this article
Omidi, F., Zangiabadian, M., Shahidi Bonjar, A.H. et al. Influenza vaccination and major cardiovascular risk: a systematic review and meta-analysis of clinical trials studies.
Sci Rep 13, 20235 (2023). https://doi.org/10.1038/s41598-023-47690-9
-
Received: 06 August 2023
-
Accepted: 16 November 2023
-
Published: 19 November 2023
-
DOI: https://doi.org/10.1038/s41598-023-47690-9
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.