Cardiovascular
Medtronic gains 2 key approvals for its ablation portfolio
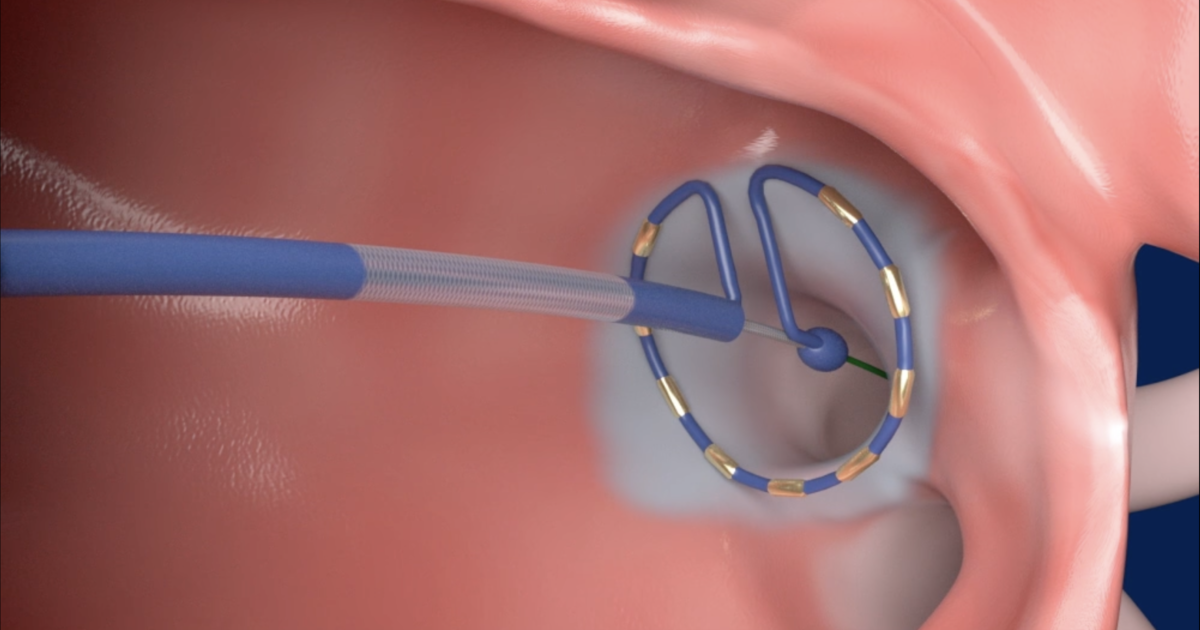
Medtronic has gained European CE mark approval for its PulseSelect Pulsed Field Ablation (PFA) System and the Nitron CryoConsole.
PulseSelect, designed to treat atrial fibrillation (AFib), is a key piece of Medtronic’s cardiac ablation portfolio. It uses PFA to target a patient’s pulmonary veins with 30-second bursts of energy. In trials, pulsed field has the same level of efficacy as traditional thermal ablation using RF or cryo-ablation. However, the safety profile is much greater, because the technology uses energy bursts to create electroporation, where holes developed in cells that lead to cell death, which is easier to control and helps prevent damage to underlying tissues such as the esophagus.
The belief is that PFA helps clinicians target AFib in a way that avoids the risks associated with thermal ablation and cryoablation. Once approved by the U.S. Food and Drug Administration (FDA), many specialists in the cardiology space think it could go on to replace the other ablation modalities for that very reason.
The Nitron CryConsole, meanwhile, houses Medtronic’s Arctic Front and Freezor cryoablation catheters. It includes a new touchscreen interface, wired remote control and features that help guide the operator through ablation procedures.