Infection
Thrombocytopenia and venous thromboembolic events after BNT162b2, CoronaVac, ChAdOx1 vaccines and SARS-CoV-2 infection: a self-controlled case series study
Abstract
This study assessed the association between COVID-19 vaccines, SARS-CoV-2 infection and the risk of thrombocytopenia and venous thromboembolism (VTE). This self-controlled case series study used hospital records between 1st February 2021 and 28th February 2022 linked to the national immunisation registry and COVID-19 surveillance data in Malaysia. Conditional Poisson regression was used to estimate incidence rate ratios (IRR) of events in the risk period (day 1–21 post-exposure) relative to control period with the corresponding 95% confidence interval (CI) adjusted for calendar period. We found no significant increased risk of thrombocytopenia in 1–21 days following BNT162b2, CoronaVac and ChAdOx1 vaccines while the risk was increased following SARS-CoV-2 infection (IRR 15.52, 95% CI 13.38–18.00). Similarly, vaccination with BNT162b2, CoronaVac, or ChAdOx1 was not associated with an increased risk of VTE during the 1–21 days risk period. SARS-CoV-2 infection was associated with increased risk of VTE (IRR 39.84, 95% CI 27.45–32.44). Our findings showed low event rates of thrombocytopenia and VTE following booster vaccination with comparable safety profiles between those who received homologous and heterologous booster combinations. Our findings showed the risk of thrombocytopenia and VTE was not increased after COVID-19 vaccination while the risks were substantially higher after SARS-CoV-2 infection.
Introduction
Since the global deployment of COVID-19 vaccines, billions of doses have been administered to the population worldwide to combat the COVID-19 pandemic. Whilst COVID-19 vaccines authorised for use have been shown to be safe and effective1,2,3, these vaccines continue to be monitored in the post-marketing phase to gather data on safety profile. Two of the most serious side effects, thrombocytopenia and thrombosis, were first reported to regulatory authorities in the first quarter of 2021 following rare cases that occurred shortly after COVID-19 vaccination4,5. Cases were mostly linked to the adenoviral vector-based vaccines, ChAdOx1 nCoV-19 (AstraZeneca) and Ad26.COV2.S (Janssen), where the vaccine appear to stimulate autoantibodies to platelet factor 4 (PF4), resulting in platelet activation6,7,8,9. The condition also referred to as thrombosis with thrombocytopenia syndrome, appeared to be a rare side effect of the vector-based COVID-19 vaccine4,10,11. This resulted in several countries pausing or limiting the use of ChAdOx1 and Ad26.COV2-S vaccine.
Cases of thrombosis or thrombocytopenia following administration of mRNA-based vaccine were similarly observed, but at a smaller extent compared to ChAdOx1 with less conclusive findings12,13,14. Data concerning the association of these events with inactivated COVID-19 vaccine remains scarce, with limited studies that described the risk for CoronaVac. Of note, different vaccine platforms have variations in their safety profiles since the mechanisms to trigger an immune response are different. Population-based studies thus far predominantly from Western countries that described the risk of events in the population with most comparisons between mRNA and adenoviral vector COVID-19 vaccine. Limited evidence is available on whether such risks are similarly observed in Asian populations, given that differences exist in the genetic and risk factors between Western and Asian populations that may potentially affect the vaccine safety profile15,16. Furthermore, evidence suggests that SARS-CoV-2 infection is a risk factor for thrombosis and thrombocytopenia12,17,18,19. Therefore, a comparison of the risk of these events after SARS-CoV-2 vaccination and SARS-CoV-2 infection will provide context for risk assessment.
In Malaysia, the national immunisation program against COVID-19 commenced in February 2021 and since then, more than 70 million doses of COVID-19 vaccines have been administered with over 80% of the adult population being fully vaccinated as of June 202220. Malaysia primarily used the mRNA vaccine BNT162b2 (Pfizer BioNTech), inactivated vaccine CoronaVac (Sinovac), and adenoviral vector vaccine ChAdOx1 (AstraZeneca), while other vaccines approved for use in Malaysia were administered to less than 5% of the population. We conducted a population-based study to evaluate the risk of thrombocytopenia and venous thromboembolism (VTE) with the BNT162b2, CoronaVac, and ChAdOx1 vaccines. Similarly, we assessed the risk of these outcome events following SARS-CoV-2 infection for comparison with the risk after vaccination.
Materials and methods
Study design
We used self-controlled case series (SCCS) method to measure the risks of thrombocytopenia and VTE after COVID-19 vaccination and SARS-CoV-2 infection. The SCCS determines the relative incidence of outcome for specific risk periods compared to control periods in individuals with the outcome of interest, in which persons act as their own controls and eliminate time-fixed confounding21,22. This method has been widely used and recommended for vaccine safety monitoring23.
Data sources
Data on hospitalisations were retrieved from the Malaysian Health Data Warehouse, a national administration database that captures services and visits to healthcare facilities across Malaysia24. Hospital inpatient episodes contain details of patient information, admission and discharge dates, and diagnoses coded using the International Classification of Diseases 10th Revision (ICD-10). We used data from public hospitals, which cover approximately 70% of all hospital admissions in Malaysia25, due to poor data linkage ability for data from private hospitals. Individual-level COVID-19 vaccination data was extracted from the Malaysia Vaccine Administration System (MyVAS), a nationwide database that captured all COVID-19 vaccinations in Malaysia and provided information on the date, type, and dose of the administered vaccines. This database also includes details for individuals who received COVID-19 vaccination outside Malaysia, whose vaccination records were captured upon entry to Malaysia as part of the national requirements. Additionally, the national COVID-19 surveillance system which includes a registry of all patients with a confirmed SARS-CoV-2 infection in Malaysia provided information on COVID-19 diagnosis status (diagnostic test, date of positive test). These databases contain unique individual identification number which was used for data linkage using exact matching. Linked data were de-identified for further analysis.
Exposure
The main exposure was COVID-19 vaccination, defined as receipt of BNT162b2, CoronaVac, and ChAdOx1. Other vaccines were less frequently used in the population in Malaysia and not included in the current analysis. The date of the vaccination was used as the exposure date. For individuals who received multiple vaccine doses during the observation period, each dose was considered separately. In Malaysia, the recommended vaccination dose schedule between the first and second dose is 21 days for BNT162b2 and CoronaVac and 4–12 weeks for ChAdOx126. Individuals are eligible to receive a third dose, hereafter referred to as a booster dose, after a minimum of 90 days after completion of the primary dose schedule. Heterologous booster refers to receipt of a different vaccine brand than the primary dose series, while homologous booster refers to receipt of the same vaccine brand for the booster and primary dose series. Individuals were grouped by vaccine types and doses received. The secondary exposure was SARS-CoV-2 infection, defined as a positive test result for SARS-CoV-2. The date of tested positive was used as the exposure date.
Outcomes
Study outcomes were hospital admission associated with thrombocytopenia and VTE, considered separately. VTE include a composite of pulmonary embolism, lower limb venous thrombosis, splanchnic thrombosis, and cerebral venous sinus thrombosis. Events were identified using ICD-10 codes from the diagnosis fields. The list of ICD-10 codes used for case identification is available in Appendix 1. Only the first event recorded during follow-up were included.
Study population
We identified individuals admitted to hospitals with diagnoses of thrombocytopenia and VTE between 1 February 2021 and 28 February 2022, the study observation period, as cases. For each outcome, we excluded individuals who had a documented hospitalisation for the outcome diagnoses in the two years before the study period.
Vaccinated cohort comprised cases who had received at least one dose of BNT162b2, CoronaVac, or ChAdOx1. We excluded individuals from the vaccinated cohort if they (i) received any other COVID-19 vaccine brand, (ii) had a mixture of COVID-19 vaccine brands for the primary vaccination series (dose 1 and dose), and (iii) did not follow the dosing schedule. Those who had a positive SARS-CoV-2 test result in ≤ 30 days before or during admission for the outcome event were also excluded since COVID-19 can also increase the risk of outcome events18,27. We used 30 days to eliminate the risk during the acute infection period.
To study the association between SARS-CoV-2 infection and outcomes, SARS-CoV-2 infection cohort comprised of cases with a documented SARS-CoV-2 positive test result between 1 February 2021 and 28 February 2022. Individuals who received a COVID-19 vaccine dose in ≤ 30 days before or during admission for the outcome event were excluded from analysis of the infected cohort to eliminate risk potentially associated with the COVID-19 vaccination.
Statistical analysis
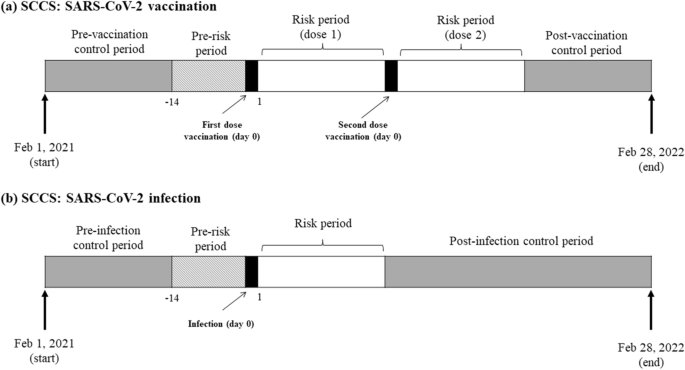
Analysis was conducted separately for each exposure and outcome of interest. Follow-up was from the start of the study period (1 February 2021) until 28 February 2022. The SCCS models were fitted using conditional Poisson regression model with an offset of the length of risk periods. Time windows were defined relative to the vaccination or infection date (Fig. 1). Exposure risk period was defined as 21 days following each exposure date (vaccination date or date of positive infection). The 21-day risk period was selected based on the vaccine dose schedule and published literature, which is considered sufficient to identify risk of acute events following vaccination or infection18,28,29. The day of exposure was considered separately (day 0). All other observation periods are considered as the reference period (control).
Follow-up of patients in self-controlled case series study for (a) COVID-19 vaccinated cohort and (b) COVID-19 infected cohort. Abbreviation: SCCS, self-controlled case series.
The models were adjusted for months in the observation period to account for potential factors associated with calendar time. Incidence rate ratios (IRRs) of events in the risk period relative to the control periods were calculated with the corresponding 95% confidence interval (CI) for each model. A 95% CI that did not include one indicates statistical significance. Each dose of vaccination was considered as a separate risk window to account for the dose effect. We excluded the booster dose from the SCCS model due to the low number of cases over the observation period to estimate them reliably. Follow-up for SCCS was to the end of the study period (28 February 2022) or censored upon receipt of a booster dose. The incidence of thrombocytopenia and VTE within 21 days after receiving a COVID-19 vaccine booster dose was presented descriptively as crude absolute risk over total doses administered.
Subgroup analyses were conducted by age and sex to explore any age or sex effect. Patient age was divided into the following categories: 18–39 years and ≥ 40 years. These age categories were used based on restricted recommendations for ChAdOx1 vaccine in those younger than 40 years old in several countries due to potential risk of blood clots and low platelets in the younger population30,31. VTE cases were also analysed by the types of thrombosis. A 14-day pre-risk period before exposure was removed from the control period and reported separately to account for potential bias if the outcome influenced the likelihood of exposure (event-dependent exposure). Another assumption of the SCCS method is that events must be independent of one another; therefore, analysis was restricted to only the first event. In a sensitivity analysis, we excluded fatal events to check for event-dependent censoring of observation time. Several other sensitivity analyses were conducted to check for robustness of the results: (i) including only pre-exposure period as the control period, and (ii) excluding individuals with any documented SARS-CoV-2 positive test before and during the study period. Cell numbers with values fewer than three were blinded and reported as less than three (< 3). Analyses were performed using STATA SE version 15 (StataCorp, College Station, Tx, USA).
Ethical approval
This study was part of the project “Case-based monitoring of adverse events following COVID-19 vaccination (SAFECOVAC)” that received approval from the Medical Research and Ethics Committee, Ministry of Health Malaysia (NMRR-21-322-59745) which include waiver of informed consent due to the use of secondary data for this research.
Results
As of 28 February 2022, 90% of the adult population in Malaysia had been vaccinated against SARS-CoV-2 and over 14 million booster doses were administered to the population, while there were over 3 million (3,227,777) SARS-CoV-2 positive cases were recorded. Between 1 February 2021 and 28 February 2022, we identified a total of 7116 thrombocytopenia and 12,302 VTE that were hospitalised. The selection of cases for inclusion in the SCCS analysis is summarised in Fig. 2.

Flowchart of selection of (a) thrombocytopenia and (b) venous thromboembolism cases for analysis. Abbreviation: SCCS, self-controlled case series.
Table 1 shows characteristics of cases that met the eligibility criteria and grouped by COVID-19 vaccinated and infected cohorts. Among individuals who were vaccinated, there were 3330 thrombocytopenia and 4124 VTE cases. Majority (65%) had received BNT162b2 vaccine, while approximately 30% and 5% of them received CoronaVac and ChAdOx1 vaccines, respectively.
Of those included in the SARS-CoV-2 infected cohort, case counts were as follows: thrombocytopenia 1236, VTE 4193. Of the thrombocytopenia cases, 3189 cases (95.0%) from the vaccinated group were discharged alive compared to 88.5% in those infected with SARS-CoV-2. Among those who had VTE, cases that were discharged alive were 91.7% in the vaccinated group and 75.6% in those with SARS-CoV-2 infection.
Thrombocytopenia
A total of 344 cases of thrombocytopenia occurred within 21 days after the first and second doses of BNT162, CoronaVac, and ChAdOx1 vaccines. The number of events and adjusted IRRs for each risk period by vaccine types and doses are summarised in Table 2. There was no increased risk of thrombocytopenia during 1 to 21 days after vaccination with BNT162b2 and CoronaVac compared to the control period. Results are consistent for both the first and second doses. The adjusted IRRs for ChAdOx1 were slightly elevated with values above 1, but the results were not significant (first dose, IRR 1.36; 95% CI 0.67–2.78; second dose, IRR 1.45; 95% CI 0.68–3.10). None of the strata showed a significantly higher risk of thrombocytopenia after vaccination when the risk period was subdivided into separate weeks.
Thrombocytopenia was observed in 381 patients within 21 days following a positive SARS-CoV-2 test, resulting in an adjusted IRR of 15.52 (95% CI 13.38–18.00) (Table 2). The risk was highest during the first week following infection (IRR 38.09; 95% CI 32.64–44.46).
Venous thromboembolism
The risk of VTE was found to be not significantly increased during the 1–21 days after both the first and second doses of BNT162b2, CoronaVac, and ChAdOx1 (Table 2). There was some evidence of increased risk of VTE associated with ChAdOx1 when the risk period was subdivided by weeks since vaccination, where the risk estimates during day 8–14 after the first dose of ChAdOx1 was 2.74 (1.37–5.49).
In the 21-day risk period following SARS-CoV-2 infection, there were 1929 cases of VTE. A significantly increased risk of VTE was observed during the risk period (day 1–21) compared to the control period with IRR of 29.84 (95% CI 27.45–32.44). Analysis by week showed that the risk was highest during the first week following infection (IRR 64.99; 95% CI 59.60–70.89).
Subgroup and sensitivity analyses
Consistent with the results of the main analysis, subgroup analyses stratified by age groups and sex did not show significant association between the outcome events and vaccination with BNT162b2, CoronaVac, or ChAdOx1 within the 21-day risk period after vaccination (Table 3). The risk of VTE after the first dose of ChAdOx1 was two-fold higher in females than males, but it was not statistically significant. Increased risk of thrombocytopenia and VTE following SARS-CoV-2 infection was similarly observed when analysis was stratified by age and sex but we did not observe any difference in the risk across the subgroup. Subgroup analysis by types of VTE did not result in significant IRR (Appendix 2).
Results of the sensitivity analyses remain largely similar to the main analysis and confirm the robustness of the results. Exclusion of fatal events from the SCCS model showed no association between COVID-19 vaccination and the risk of thrombocytopenia and VTE (Appendix 3). When we restricted the control period to only include the pre-exposure period (Appendix 4), the risks increased slightly particularly for thrombocytopenia following the second dose of BNT162b2 and CoronaVac vaccines, but stayed non-significant. Exclusion of cases with any history of COVID-19 diagnosis (Appendix 5) showed estimates that were consistent with the main analysis, but with wider confidence intervals due to the smaller number of cases included.
Risk of events after COVID-19 vaccine booster dose
Table 4 shows the number of thrombocytopenia and VTE that were observed following the administration of COVID-19 vaccine booster dose. Overall, there were 58 cases of thrombocytopenia and 67 cases of VTE within the first 21 days after receipt of the booster dose. The absolute risk of the event ranged from three to six cases for every one million booster vaccine doses administered. Most cases were reported among recipients of BNT162b2 as booster dose irrespective of the primary vaccination series. The absolute risks of thrombocytopenia within 21 days after booster vaccination for heterologous and homologous booster groups were 4.5 (95% CI 3.1–6.3) and 3.4 (95% CI 2.2–5.0) per 1 million doses, respectively. For VTE, the absolute risk in heterologous booster was 4.9 (95% CI 3.5–6.8) compared to 4.2 (95% CI 2.8–6.0) in homologous group.
Discussion
This study estimated the risk of hospitalisation for thrombocytopenia and VTE following SARS-CoV-2 vaccination and infection. Risk estimates for both thrombocytopenia and VTE during the 21-day risk period were > 1 following exposure to ChAdOx1 vaccine, but were not statistically significant. No increased risk was observed following BNT162b2 and CoronaVac. A substantially higher risk of both thrombocytopenia and VTE was observed after SARS-CoV-2 infection.
Previous studies showed an increased risk of thrombocytopenia, VTE, and cerebral venous sinus thrombosis following COVID-19 vaccination, particularly with ChAdOx112,14,32,33. Recently, Berild et al.13 reported elevated risk of thrombocytopenia and thromboembolic events after ChAdOx1 in an SCCS analysis that pooled data from Norway, Finland, and Denmark. Notably, these studies only studied the first dose of vaccine due to the limited number of people receiving second dose during the study period. Our findings included analysis by first and second dose which showed that there was an indication of slightly increased risk of thrombocytopenia and VTE among people receiving ChAdOx1 vaccine, but the association was not statistically significant. We did find a significant association between ChAdOx1 and VTE during day 8–14 after the first dose (IRR 2.74; 95% CI 1.37–5.49), which corresponds to other studies that found the risk to be higher around week 2 after vaccination12,34. Nevertheless, the numbers were small and further confirmation is needed. Our study cohort represents an Asian population of multi-ethnicity which was slightly different than previous studies that were predominantly Caucasian. Furthermore, the vaccination policy in Malaysia has to be taken into account whereby ChAdOx1 vaccine was initially given as voluntary opt-in due to safety concerns during the roll-out3. ChAdOx1 vaccine was well-received among the younger population due to its availability before their originally scheduled vaccination appointments3. Therefore, people who received ChAdOx1 represent a younger and likely healthier population. In this study, we did not find significant association between BNT162b2 and the outcome events. This broadly echoes other studies that reported no statistically significant risk for thrombocytopenia and thromboembolic events in the 3–4 weeks following BNT162b2 vaccination14,33. Our findings further showed no evidence of association for such events among people who received CoronaVac which aligned with published studies from Hong Kong35,36.
Many countries, including Malaysia, introduced a booster dose of COVID-19 vaccine in fully vaccinated recipients to enhance waning induced immunity and immunity against new SARS-CoV-2 variants37. In our study, thrombocytopenia and VTE that occurred during 1 to 21 days after receipt of COVID-19 vaccine booster accounted for less than ten cases for every one million booster doses administered. Cases were mostly reported for BNT162b2 which corresponds to the country’s COVID-19 vaccine booster coverage since BNT162b2 was the primary vaccine administered as a booster shot to the population while another vaccine type was offered as an alternative to BNT162b2. Our findings showed comparable safety profiles between those who received homologous and heterologous booster combinations, which provides reassurance on the safety of these booster combinations.
While the concern of post-vaccination thrombosis is one of the reasons for vaccine hesitancy in many, the present analysis has shown contrarily that the risk of developing these thrombotic events following SARS-CoV2 infection itself was way higher than the risk from COVID-19 vaccination. Our findings hence support other studies where high rates of thrombocytopenia and VTE were encountered in patients with SARS-CoV2 infection, than after a COVID-19 vaccine in the same population12,38,39,40. The reason for this is that COVID-19 disease may itself be associated with coagulation dysfunction which predisposes patients to an elevated risk of thromboembolism40,41. However, the specific mechanism(s) for the increased incidence of venous thrombosis among patients with SARS-CoV2 infection has not been fully elucidated. Importantly, the risks of both thrombocytopenia and VTE after COVID-19 vaccination were much lower than those associated with SARS-CoV-2 infection.
Strength and limitation
Our study provided real-world evidence on the safety of COVID-19 vaccination with direct comparisons between the three different vaccine platforms within the same population. Particularly, we provided evidence on the safety profiles of inactivated CoronaVac vaccine, which are still limited compared to other platforms despite accounting for nearly 50% of the COVID-19 vaccine doses delivered globally42. Compared to existing evidence from studies conducted mainly in Western countries, our cohort consists of an Asian population administered different types of COVID-19 vaccines, including administration of both homologous and heterologous booster doses. We used large, nationwide database to provide population-based safety data on the risks associated with both COVID-19 vaccination and infection. Linkage to national databases of COVID-19 vaccination and COVID-19 cases minimises the bias from misclassifying exposure.
There are several limitations in this study. First, the outcome events were based on hospital admissions. We did not include cases that were treated in primary care, outpatient, or those who died before being admitted. Second, we did not include data from private hospitals. Nevertheless, public hospitals cover approximately 70% of all hospital admissions in the country43; therefore, we expect the cases that were not captured to be relatively small. Third, case ascertainment was based on diagnosis codes of hospital records which might overestimate the risk without including other laboratory parameters to define and validate the case. Fourth, we do not have data on medication history or pharmacological treatment provided during hospitalisation. Therefore, we cannot adjust for inherent factors pertaining to individuals who might be on certain treatments that could affect the risk profile. Similarly, we do not have data on individuals’ comorbidities and therefore, we are unable to account for the effect of existing medical conditions on the association measured in this study. To minimise this bias, we excluded individuals who had experienced the outcome in the 2 years before the index hospitalisation and included only the first event that occurred during the study period for analysis. Next, with the use of secondary databases, we cannot completely rule out misclassification bias and unmeasured confounding, such as smoking, alcohol consumption, body mass index, or disease severity. These factors are known to be common risk factors for the outcome, but we did not have the information in the database that we used. Therefore, further studies will have to address these information gaps and risk factors not adjusted for in this study. Lastly, some of the subgroup analyses present a small sample size and results should be interpreted with caution.
Policy implications
The finding on the risk of thrombocytopenia and thrombotic complications after COVID-19 vaccines and after SARS-CoV-2 infection will be useful in the risk–benefit assessment for vaccine-related policies or purposes and public health decision-making as well as in providing quantification of the risk of thrombotic events associated with COVID-19 vaccines to the general public. This also emphasises the need for clinicians to implement thromboprophylaxis protocols to reduce the risk of thromboembolism among SARS-CoV-2 infected individuals. The findings of the present study also provided some insights into the safety profile of booster doses of COVID-19 vaccination. This information may help address vaccine hesitancy, which is a key challenge for public health regarding containing infectious diseases and pandemic prevention.
Conclusion
Among our study population, the risks of thrombocytopenia and VTE were not increased during 1–21 days after COVID-19 vaccination compared with the control period. The risks of these events were higher after vaccination with ChAdOx1 compared to BNT162b2 and CoronaVac, although the increase in risk was not significant. The rates of both thrombocytopenia and VTE after booster doses were generally low after either vaccine. However, SARS-CoV-2 infections were associated with a significantly increased risk of both thrombocytopenia and VTE in the same population. The benefit-risk profiles of COVID-19 vaccination pertaining to these events should be interpreted in light of current findings to guide decision-making for vaccination to protect against complications of SARS-CoV-2 infection.
Data availability
The data that supports the findings of this study are available within the article and its supplementary materials. Access to datasets is provided by the corresponding data custodians for analysis for this study, but we have no permission to make generated datasets available. Malaysia COVID-19 vaccine administration data are available at https://github.com/MoH-Malaysia/covid19-public, redacted of personal identifying information.
References
-
Polack, F. P. et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 383(27), 2603–2615 (2020).
Google Scholar
-
Voysey, M. et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 397(10269), 99–111 (2021).
Google Scholar
-
Suah, J. L. et al. PICK-ing Malaysia’s epidemic apart: Effectiveness of a diverse COVID-19 vaccine portfolio. Vaccines (Basel) 9(12), 1381 (2021).
Google Scholar
-
European Medicines Agency. COVID-19 Vaccine AstraZeneca: Benefits still outweigh the risks despite possible link to rare blood clots with low platelets 2021 [1 June 2022]. Available from: https://www.ema.europa.eu/en/news/covid-19-vaccine-astrazeneca-benefits-still-outweigh-risks-despite-possible-link-rare-blood-clots.
-
Pottegård, A. et al. Arterial events, venous thromboembolism, thrombocytopenia, and bleeding after vaccination with Oxford-AstraZeneca ChAdOx1-S in Denmark and Norway: Population based cohort study. bmj 373, n1114 (2021).
Google Scholar
-
Greinacher, A. et al. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N. Engl. J. Med. 384(22), 2092–2101 (2021).
Google Scholar
-
Schultz, N. H. et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N. Engl. J. Med. 384(22), 2124–2130 (2021).
Google Scholar
-
Muir, K. L., Kallam, A., Koepsell, S. A. & Gundabolu, K. Thrombotic thrombocytopenia after Ad26.COV2.S vaccination. N. Engl. J. Med. 384(20), 1964–1965 (2021).
Google Scholar
-
Scully, M. et al. Pathologic antibodies to platelet factor 4 after ChAdOx1 nCoV-19 vaccination. N. Engl. J. Med. 384(23), 2202–2211 (2021).
Google Scholar
-
World Health Organisation (WHO). Guidance for clinical case management of thrombosis with thrombocytopenia syndrome (TTS) following vaccination to prevent coronavirus disease (COVID-19). Interim guidance 2021 [12 June 2022]. Available from: https://apps.who.int/iris/bitstream/handle/10665/342999/WHO-2019-nCoV-TTS-2021.1-eng.pdf?sequence=1&isAllowed=y.
-
National Pharmaceutical Regulatory Agency. Ministry of Health Malaysia. Safety updates on COVID-19 vaccines: Risk of vaccine-induced immune thrombotic thrombocytopenia (VITT)/thrombosis with thrombocytopenia syndrome (TTS) 2022 [12 June 2022]. Available from: https://npra.gov.my/index.php/en/component/content/article/435-english/safety-alerts-main/safety-alerts-2022/1527349-safety-updates-on-covid-19-vaccines-risk-of-vaccine-induced-immune-thrombotic-thrombocytopenia-vitt-thrombosis-with-thrombocytopenia-syndrome-tts.html?Itemid=1391.
-
Hippisley-Cox, J. et al. Risk of thrombocytopenia and thromboembolism after covid-19 vaccination and SARS-CoV-2 positive testing: self-controlled case series study. bmj 374, n1931 (2021).
Google Scholar
-
Dag Berild, J. et al. Analysis of thromboembolic and thrombocytopenic events after the AZD1222, BNT162b2, and MRNA-1273 COVID-19 vaccines in 3 Nordic countries. JAMA Netw. Open 5(6), e2217375 (2022).
Google Scholar
-
Whiteley, W. N. et al. Association of COVID-19 vaccines ChAdOx1 and BNT162b2 with major venous, arterial, or thrombocytopenic events: A population-based cohort study of 46 million adults in England. PLoS Med. 19(2), e1003926 (2022).
Google Scholar
-
White, R. H. & Keenan, C. R. Effects of race and ethnicity on the incidence of venous thromboembolism. Thromb. Res. 123(Suppl 4), S11–S17 (2009).
Google Scholar
-
Ho, F. K. et al. Ethnic differences in cardiovascular risk: Examining differential exposure and susceptibility to risk factors. BMC Med. 20(1), 149 (2022).
Google Scholar
-
Ho, F. K. et al. Thromboembolic risk in hospitalized and nonhospitalized COVID-19 patients: A self-controlled case series analysis of a nationwide cohort. Mayo Clin. Proc. 96(10), 2587–2597 (2021).
Google Scholar
-
Katsoularis, I. et al. Risks of deep vein thrombosis, pulmonary embolism, and bleeding after covid-19: Nationwide self-controlled cases series and matched cohort study. bmj 377, e069590 (2022).
Google Scholar
-
Voigtlaender, M. et al. Thromboembolic events in deceased patients with proven SARS-CoV-2 infection: Frequency, characteristics and risk factors. Thromb. Res. 218, 171–176 (2022).
Google Scholar
-
Ministry of Health Malaysia. Vaccination in Malaysia [31 March 2022]. Available from: https://covidnow.moh.gov.my.
-
Whitaker, H. J., Farrington, C. P., Spiessens, B. & Musonda, P. Tutorial in biostatistics: The self-controlled case series method. Stat. Med. 25(10), 1768–1797 (2006).
Google Scholar
-
Petersen, I., Douglas, I. & Whitaker, H. Self controlled case series methods: An alternative to standard epidemiological study designs. bmj 354, i4515 (2016).
Google Scholar
-
Weldeselassie, Y. G., Whitaker, H. J. & Farrington, C. P. Use of the self-controlled case-series method in vaccine safety studies: Review and recommendations for best practice. Epidemiol. Infect. 139(12), 1805–1817 (2011).
Google Scholar
-
Ministry of Health Malaysia. Malaysian Health Data Warehouse [Available from: https://myhdw.moh.gov.my/public/home.
-
Ministry of Health Malaysia. Health Facts 2022 [Available from: www.moh.gov.my].
-
Ministry of Health Malaysia. Clinical Guidelines on COVID-19 Vaccination in Malaysia 4th edn (2021).
-
Burn, E. et al. Venous or arterial thrombosis and deaths among COVID-19 cases: A European network cohort study. Lancet Infect. Dis. 22(8), 1142–1152 (2022).
Google Scholar
-
Klein, N. P. et al. Surveillance for adverse events after COVID-19 mRNA vaccination. JAMA 326(14), 1390–1399 (2021).
Google Scholar
-
Wong, C. K. H. et al. Adverse events of special interest and mortality following vaccination with mRNA (BNT162b2) and inactivated (CoronaVac) SARS-CoV-2 vaccines in Hong Kong: A retrospective study. PLoS Med. 19(6), e1004018 (2022).
Google Scholar
-
COVID-19 vaccines: JCVI recommendations on the use of the AstraZeneca vaccine [Available from: https://www.gov.uk/government/publications/use-of-the-astrazeneca-covid-19-vaccine-jcvi-statement-7-may-2021.
-
Pugh, J., Wilkinson, D., Kerridge, I. & Savulescu, J. Vaccine suspension, risk, and precaution in a pandemic. J. Law Biosci. 9(1), lsab036 (2022).
Google Scholar
-
Kerr, S. et al. First dose ChAdOx1 and BNT162b2 COVID-19 vaccinations and cerebral venous sinus thrombosis: A pooled self-controlled case series study of 11.6 million individuals in England, Scotland, and Wales. PLoS Med. 19(2), e1003927 (2022).
Google Scholar
-
Simpson, C. R. et al. First-dose ChAdOx1 and BNT162b2 COVID-19 vaccines and thrombocytopenic, thromboembolic and hemorrhagic events in Scotland. Nat. Med. 27(7), 1290–1297 (2021).
Google Scholar
-
Higgins, H. et al. Risk of thrombosis with thrombocytopenia syndrome after COVID-19 vaccination prior to the recognition of vaccine-induced thrombocytopenia and thrombosis: A self-controlled case series study in England. Res. Pract. Thromb. Haemost. 6(3), e12698 (2022).
Google Scholar
-
Sing, C. W. et al. COVID-19 vaccines and risks of hematological abnormalities: Nested case-control and self-controlled case series study. Am. J. Hematol. 97(4), 470–480 (2022).
Google Scholar
-
Chui, C. S. L. et al. Thromboembolic events and hemorrhagic stroke after mRNA (BNT162b2) and inactivated (CoronaVac) Covid-19 vaccination: A self-controlled case series study. EClinicalMedicine 50, 101504 (2022).
Google Scholar
-
Suah, J. L. et al. Real-world effectiveness of homologous and heterologous BNT162b2, CoronaVac, and AZD1222 booster vaccination against Delta and Omicron SARS-CoV-2 infection. Emerg. Microbes Infect. 11(1), 1343–1345 (2022).
Google Scholar
-
Nopp, S., Moik, F., Jilma, B., Pabinger, I. & Ay, C. Risk of venous thromboembolism in patients with COVID-19: A systematic review and meta-analysis. Res. Pract. Thromb. Haemost. 4(7), 1178–1191 (2020).
Google Scholar
-
Tan, B. K. et al. Arterial and venous thromboembolism in COVID-19: A study-level meta-analysis. Thorax 76(10), 970–979 (2021).
Google Scholar
-
Llitjos, J. F. et al. High incidence of venous thromboembolic events in anticoagulated severe COVID-19 patients. J. Thromb. Haemost. 18(7), 1743–1746 (2020).
Google Scholar
-
Kipshidze, N. et al. Viral coagulopathy in patients with COVID-19: Treatment and care. Clin. Appl. Thromb. Hemost. 26, 1076029620936776 (2020).
Google Scholar
-
Mallapaty, S. China’s COVID vaccines have been crucial—Now immunity is waning. Nature 598(7881), 398–399 (2021).
Google Scholar
-
Ministry of Health Malaysia. Health Facts 2019 (2019).
Acknowledgements
The authors thank the Director General of Health Malaysia for his permission to publish this study. We thank all investigators and collaborators for their immense contribution and support. We would also like to acknowledge all data providers who make data available for research and research assistants who were involved in data collection. Members of the SAFECOVAC study group are listed in Appendix 6.
Funding
This research was supported by a grant from the Ministry of Health Malaysia—Sukuk Prihatin (NMRR-21-822-59745). The funding body had no role in the study design, data analysis, or the presentation of data and writing of the manuscript.
Author information
Authors and Affiliations
Consortia
Contributions
N.A.R.: Conceptualisation, Methodology, Formal analysis, Writing—original draft, Visualisation, Funding acquisition. M.T.L. and F.Y.L.: Investigation, Writing—review and editing, Project administration. W.K.W., H.S.Y.: Investigation, Writing—review and editing. K.P. and S.S.: Conceptualisation, Supervision, Funding acquisition, Writing—review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary Information.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
Reprints and Permissions
About this article
Cite this article
Ab Rahman, N., Lim, M.T., Lee, F.Y. et al. Thrombocytopenia and venous thromboembolic events after BNT162b2, CoronaVac, ChAdOx1 vaccines and SARS-CoV-2 infection: a self-controlled case series study.
Sci Rep 13, 20471 (2023). https://doi.org/10.1038/s41598-023-47486-x
-
Received: 18 October 2022
-
Accepted: 14 November 2023
-
Published: 22 November 2023
-
DOI: https://doi.org/10.1038/s41598-023-47486-x
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.