Cardiovascular
Effects of temperature and humidity on cerebrovascular disease hospitalization in a super-aging society
Abstract
Weather conditions influence the incidence of cardiovascular disease. However, few studies have investigated the association between weather temperature and humidity and cerebrovascular disease hospitalizations in a super-aging society. We included 606,807 consecutive patients with cerebrovascular disease admitted to Japanese acute-care hospitals between 2015 and 2019. The primary outcome was the number of cerebrovascular disease hospitalizations per day. Multilevel mixed-effects linear regression models were used to estimate the association of mean temperature and humidity, 1 day before hospital admission, with cerebrovascular disease hospitalizations, after adjusting for air pollution, hospital, and patient demographics. Lower mean temperatures and humidity < 70% or humidity ≧ 70% are associated with an increased incidence of cerebrovascular disease hospitalization (coefficient, − 1.442 [− 1.473 to − 1.411] per °C, p < 0.001, coefficient, − 0.084 [− 0.112 to − 0.056] per%, p < 0.001, and coefficient, 0.136 [0.103 to 0.168] per %, p < 0.001, respectively). Lower mean temperatures and extremely lower or higher humidity are associated with an increased incidence of cerebrovascular disease hospitalization in a super-aging society.
Introduction
Cerebrovascular disease is one of the leading causes of death and disability in the elderly worldwide1. Recently, the incidence of hospitalization due to cerebrovascular disease has increased among older patients2, with older age being recognized as a risk factor for cerebrovascular disease development. Japan’s transition into a super-aging has resulted in a growing number of its individuals developing cerebrovascular diseases3. Consequently, the prevention of cerebrovascular disease has taken on paramount importance in maintaining a high quality of life in older age.
The incidence of cerebrovascular disease is likely associated with ambient temperature, as suggested from a small number of studies from various countries4,5. Conversely, studies have also failed to establish temperature and humidity as significant risk factors of cerebrovascular disease. While weather is a recognized predictor of cardiovascular disease6,7, limited evidence exists regarding its relationship with cerebrovascular disease in Japan. Furthermore, it remains uncertain whether temperature, humidity, or related factors play a role in influencing cerebrovascular disease incidence, particularly in Japan. Moreover, existing guidelines provide only minimal recommendations regarding temperature and humidity as risk factors of cerebrovascular disease8,9.
Therefore, we conducted an observational study using a nationwide registry database to investigate whether the number of cardio-cerebrovascular disease hospitalizations was related to weather, temperature, and humidity in a super-aging society in Japan. The Japanese Registry of All Cardiac and Vascular Diseases (JROAD) database includes all patients with cardio cerebrovascular diseases who require hospitalization and constitutes a nationwide dataset in Japan. This study aimed to assess the relationship between temperature or humidity and the development of cerebrovascular disease to guide clinicians on weather recommendations and improve healthcare for an increasingly aging society.
Results
Data were collected for 606,807 consecutive patients with cerebrovascular disease admitted to 715 acute care hospitals in Japan between 2015 and 2019. The patient characteristics and demographics are shown in Table 1.
In this study population, the median age was 75.0 (66.0–83.0) years, and 55.8% of the participants were male. The incidence rates of ischemic stroke, cerebral hemorrhage, and subarachnoid hemorrhage were 69.6%, 23.2%, and 7.3%, respectively. The number of cerebrovascular diseases overlapped in subgroups. The median Charlson Comorbidity Index score was 2.0 (1.0, 3.0). The median mean temperature and humidity 1 day before hospital admission for stroke were 17.2 °C and 69%, respectively.
Association between weather conditions and cerebrovascular disease hospitalizations
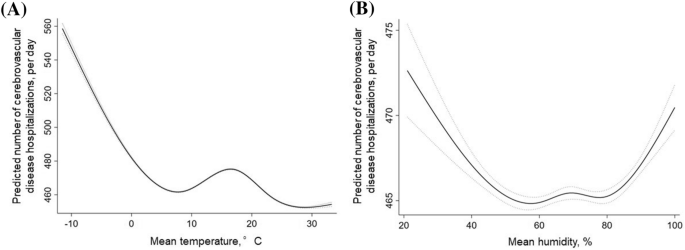
Multilevel mixed-effects linear regression analysis indicated that many incident cerebrovascular disease hospitalizations were associated with lower average temperature (coefficient, − 1.442 [− 1.473 to − 1.411] per °C (P < 0.001) after adjustments for season, PM2.5, hospital, and patient characteristics (age, sex, height, weight, smoking, and Charlson Comorbidity Index) (Table 2). The association between mean temperature and mean humidity for specific risk estimates of cerebrovascular disease incidence is summarized in Fig. 1. The number of cerebrovascular disease hospitalizations was higher with temperatures < 7 °C (coefficient, − 3.405 [− 3.547 to − 3.263]) (Fig. 1a).
Association between mean temperature or humidity and cerebrovascular disease hospitalizations. (A) Linearity was checked for continuous and categorical variables using STATA’s multivariable regression splines (MVRS) command. MVRS indicated a non-linear relationship with mean temperature. The predicted number of cerebrovascular diseases per day was univariate. The x-axis represents temperature (°C) as a continuous variable. The solid and dashed lines indicate 95% confidence intervals. (B) MVRS indicated a non-linear relationship with humidity. The predicted number of cerebrovascular diseases per day was univariate. The x-axis represents humidity (%) as a continuous variable. The solid and dashed lines indicate 95% confidence intervals.
There was a non-linear relationship between humidity and the number of cerebrovascular disease hospitalizations (Fig. 1b). A negative linear association was found between mean humidity and cerebrovascular disease hospitalization at mean humidity < 70% (coefficient, − 0.084 [− 0.112 to − 0.056]). However, a positive linear association was found at mean humidity ≥ 70% (coefficient, 0.136 [0.103 to 0.168]).
Effect of weather condition indices in the subgroups
There was a non-linear relationship between temperature and the number of ischemic stroke hospitalizations (Fig. 2a). A negative linear association was found between mean temperature and ischemic stroke hospitalization at mean temperature < 7 °C (coefficient, − 2.098 [− 2.220 to − 1.976]. However, a positive linear association was found at mean temperature ≥ 7 °C (coefficient, 0.190 [0.161 to 0.219].

Association between mean temperature and subgroups of cerebrovascular disease hospitalizations. (A) Linearity was checked for continuous and categorical variables using STATA’s multivariable regression splines (MVRS) command. MVRS indicated a non-linear relationship with mean temperature. The predicted number of ischemic stroke hospitalizations per day was univariate. The x-axis represents temperature (°C) as a continuous variable. The solid and dashed lines indicate 95% confidence intervals. (B) MVRS indicated a non-linear relationship with temperature. The predicted number of cerebral hemorrhage hospitalizations per day was univariate. The x-axis represents temperature (°C) as a continuous variable. The solid and dashed lines indicate 95% confidence intervals. (C) MVRS indicated a non-linear relationship with temperature. The predicted number of cerebral hemorrhage hospitalizations per day was univariate. The x-axis represents temperature (°C) as a continuous variable. The solid and dashed lines indicate 95% confidence intervals.
There was a non-linear relationship between humidity and the number of cerebrovascular disease hospitalizations (Fig. 3a). A negative linear association was found between mean humidity and ischemic stroke hospitalization at mean humidity < 40% (coefficient, 0.165 [0.151 to 0.179]. However, a positive linear association was found at mean humidity ≥ 40% (coefficient, 0.136 [0.103 to 0.168]).

Association between mean humidity and subgroups of cerebrovascular disease hospitalizations. (A) Linearity was checked for continuous and categorical variables using STATA’s multivariable regression splines (MVRS) command. MVRS indicated a non-linear relationship with mean humidity. The predicted number of ischemic stroke hospitalizations per day was univariate. The x-axis represents humidity (%) as a continuous variable. The solid and dashed lines indicate 95% confidence intervals. (B) MVRS indicated a non-linear relationship with humidity. The predicted number of cerebral hemorrhage hospitalizations per day was univariate. The x-axis represents humidity (%) as a continuous variable. The solid and dashed lines indicate 95% confidence intervals. (C) MVRS indicated a non-linear relationship with humidity. The predicted number of cerebral hemorrhage hospitalizations per day was univariate. The x-axis represents humidity (%) as a continuous variable. The solid and dashed lines indicate 95% confidence intervals.
The number of cerebral hemorrhage hospitalizations was higher with lower mean temperatures (coefficient, − 0.142 [− 0.151 to − 0.132] (Fig. 2b) and humidities (coefficient, − 0.142 [− 0.151 to − 0.132] (Fig. 3b). Also, the number of subarachnoid hemorrhage hospitalizations was higher with lower mean temperatures (coefficient, − 0.091 [− 0.103 to − 0.079] (Fig. 2c) and higher mean humidities (coefficient, 0.009 [0.003 to 0.015]) (Fig. 3c).
Discussion
We present a contemporary analysis of nationwide data involving 606,807 patients and describe the estimated number of hospitalizations for incident cerebrovascular disease. Overall, a higher number of incident cerebrovascular hospitalizations was associated with (1) lower mean temperatures and (2) extremely lower or higher humidity. These findings highlight the importance of assessing lower mean temperatures and extreme humidity ranges as potential indicators for cerebrovascular disease risk, particularly in a super-aging society like Japan.
We found that lower mean temperatures were associated with increased hospitalization for cerebrovascular disease. Other studies have shown that cold-induced systemic hypertension10 is a risk factor associated with the renin-angiotensin system and sympathetic nerve activity, which could modulate the incidence of cerebrovascular disease hospitalization. Vascular compression of the rostral ventrolateral medulla (RVLM) is a known cause of hypertension11,12. Patients with RVLM vascular compression have shown greater variability in blood pressure during ischemic stroke13. Also, lower temperatures may be an environmental factor that causes a higher incidence of hospitalization.
Extremely lower or higher humidity was associated with increased hospitalizations due to cerebrovascular disease. However, a systematic review showed no association between daily humidity and cerebrovascular disease occurrence14. A previous study revealed that extremely lower or higher humidity correlated with the incidence of stroke15. High humidity can cause dehydration, which increases the risk of thrombosis. However, these influences may not be as strong as those of other weather conditions, such as temperature, which may be responsible for physiological changes that could increase stroke risk16. These include increased blood pressure, erythrocyte and thrombocyte counts, and blood viscosity during cold weather17. Plasma fibrinogen concentrations are also higher in older patients, especially during a cold weather18. This may partly explain why humidity is not associated with stroke occurrence.
These findings may be influenced by differences in the cerebrovascular disease type. Ischemic stroke has a higher incidence rate than cerebral or subarachnoid hemorrhage19. Our study might also show ischemic stroke as the main finding that revealed an association between a lower mean temperature, extremely lower or higher humidity, and hospitalization due to ischemic stroke. These results were consistent with previous reports15.
Elderly individuals tend to spend more time indoors due to factors such as illnesses and decreased physical function, compared to younger individuals. Therefore, there is a possibility that the impact of temperature differences may diminish with indoor activities. Nevertheless, limited research has compared indoor time between the elderly and younger age groups. Considering that the risk of illness is higher in the elderly, it is unclear whether temperature differences indoors have a significant effect on disease incidence. Although studies have reported differences in indoor and outdoor temperature and humidity20, these differences are generally consistent with seasonal trends. Specifically, in the context of Japan, it is important to note that regional variations and the presence or absence of heating and cooling systems can also influence these differences. However, there is currently no comprehensive report that has investigated these differences across all regions of Japan.
One of the limitations of our study is that we included only Japanese diagnosis procedure combination hospitals with cardiovascular beds that meet the JCS requirements. However, the JROAD is the largest retrospective study of nationwide cardiac health outcomes in Japan and constitutes a comprehensive database of epidemiological data for population-based studies. Two, retrospective studies do not determine cause and effect; however, the large sample size is a noteworthy strength of this study. Three, When patients are exposed to weather conditions different than that of the hospital they are admitted in, it could introduce bias in the results. Furthermore, this study does not include data on specific weather conditions observed at the research site, which could affect the outcomes. Finally, the predictor of cerebrovascular disease was not adjusted for hypertension.
Conclusion
Lower mean temperatures and extremely lower or higher humidity are associated with an increased incidence of cerebrovascular disease hospitalization in the aging Japanese society. The findings of this study can provide guidance to healthcare providers on advising patients to monitor weather, temperature, and humidity in a country with an increasingly aging society.
Methods
Data collection
The JROAD database is a nationwide retrospective registry. The database was designed to assess the clinical activity of each Japanese institution regarding cardiovascular care and to provide adequate feedback to teaching hospitals for improving patient care. A detailed description of the database design and methods has been previously published21. The JCS developed the JROAD database, which includes the demographics of each hospital since 2004. The JCS also developed the JROAD-DPC nationwide database, which includes data from the Japanese diagnosis procedure combination/per diem payment system (DPC/PDPS) since 2014. The DPC database is a mixed-case classification system linked with a lump-sum payment system, launched in 2002 by the Japanese Ministry of Health, Labour and Welfare22. Compared with other registry databases, the Japanese DPC database enables researchers to conduct nationwide descriptive and/or analytical epidemiology studies in a real-world clinical setting. The JROAD database started to collect data about cerebrovascular disease in 2015. The DPC database includes data on the following elements: demographics for each patient (e.g., age and sex); principal diagnoses (coded according to the International Classification of Diseases, 10th revision [ICD-10]); comorbidities at admission (ICD-10 coded); complications after admission (ICD-10 coded); procedures, including surgery, medications, and devices used during hospitalization; length of stay; discharge status; and medical expenses21,22,23,24. Various institutions using the DPC system include academic, urban, and rural hospitals21,24. All data included in this study were from hospitalized patients with clinically apparent cardio-cerebrovascular disease. We collated and used a dataset of weather variables in Japan from the Japan Meteorological Agency (https://www.jma.go.jp/jma/indexe.html). The weather variables were daily weather temperatures and humidity, including maximal and minimum values. The humidity was measured as relative humidity. Localized weather data were obtained from the weather station closest to each hospital; as the hospitals were located in all 47 prefectures of Japan, weather data were obtained from all prefectural capitals. We searched the sites of weather stations by the municipality code, which the Ministry of Internal Affairs and Communications in Japan assigns to each municipality specifically. We combined the hospital sites with the monitoring stations in each prefecture to create a unified dataset. The weather variables were merged with the DPC database using the acute hospitalization day and the municipal code provided by the Japanese Ministry of Internal Affairs and Communications (https://www.soumu.go.jp/denshijiti/code.html). We also collated and used a dataset of air pollution variables in Japan from the National Institute for Environmental Studies (http://www.nies.go.jp/db/index-e.html). The air pollution variables included hourly PM2.5.
This study included 715 hospitals and 4,998,541 consecutive patients admitted during the study period. We collected data from patients with cardio-cerebrovascular disease who required hospitalization. The exclusion criteria were as follows: cardiovascular disease hospitalization, planned hospitalization, missing temperature or humidity data, and missing patient characteristic data. After excluding 4,391,734 patients, a total of 606,807 patients with cerebrovascular disease were included in the analysis (Fig. 4).

Flowchart of patient disposition. A total of 606,807 patients who underwent cerebrovascular disease hospitalization from 715 hospitals were included. The patients with planned hospitalization were excluded because only patients with acute hospitalized cerebrovascular disease were included for analysis. The patients with cardiovascular disease were also excluded.
The study was conducted in accordance with the principles of the Declaration of Helsinki. The author designed the present study, and the study protocol was approved by the Institutional Ethics Committee of St. Marianna University of Medicine. Each hospital anonymized the patient IDs using code-change equations for the original JROAD-DPC data. The requirement for individual informed consent was waived by the institutional ethics committee of St. Marianna University of Medicine because all data were anonymized when provided by the DPC. The data was sent to the Ministry of Health, Labor, and Welfare, Japan Meteorological Agency, and the National Cerebral Cardiovascular Center managed the database. The National Cerebral and Cardiovascular Center notified the patients that their information was being collected for this study through homepages or posters at each hospital. The patients could choose to exclude this information. This study did not investigate blood tests or electrocardiography data because we used only DPC data. The method of this study cited from previous our study6,7.
Cerebrovascular disease included ischemic stroke, cerebral hemorrhage, and subarachnoid hemorrhage. These diseases were assigned the International Statistical Classification of Diseases and Related Health Problems 10th Revision (ICD-10) code, which is recorded with the “main diagnosis, admission precipitating diagnosis, most resource-consuming diagnosis,” or “second-most resource-consuming diagnosis.” The number of cerebrovascular diseases overlapped in the subgroups. Ischemic stroke was assigned the ICD-10 code I63. Cerebral hemorrhage was assigned the ICD-10 code I61. Subarachnoid hemorrhage was assigned the ICD-10 code I60. The seasons were divided into spring (March–May), summer (June–August), autumn (September–November), and winter (December-February). The mean temporaries were defined as the mean hourly temperature and humidity within a day. The weather variables on a certain day were assigned to the day before the emergency hospitalization for cerebrovascular disease. This is because hospitalization times were not available in the database. The cutoff values associated with the results were visually determined by an author (S.D) from the graph.
Design
We conducted a retrospective study using data from JROAD and JROAD-DPC and weather variables between April 1, 2015, and March 31, 2019.
Outcomes
The primary outcome was the number of cerebrovascular diseases requiring hospitalization per day.
Covariates
The mean weather temperature and humidity, as continuous variables, were adjusted for season, PM2.5, hospital demographics (East/West Japan, number of hospital beds, presence of a coronary care unit, cardiac surgery service, and board-certified cardiologist), and patient demographics (age, sex, height, weight, smoking, and Charlson Comorbidity Index). The Charlson Comorbidity Index has been developed and validated to predict the risk of mortality in longitudinal studies25. The score can be calculated from a weighted index consisting of age and the number and severity of comorbid diseases.
Statistical analysis
Patient characteristics are expressed as medians and interquartile ranges for continuous variables. Categorical variables are presented as frequencies (%). We used multilevel mixed random-effects and population-averaged linear models to evaluate the association between the number of cerebrovascular disease hospitalizations and weather variables. Multilevel mixed-effects models were used to evaluate the random effects of hospital variations (institutional codes) using “xtset” command in STATA. Experienced cardiologists considered these covariates clinically important factors. As continuous variables, the weather temperature and humidity were adjusted for covariates.
Linearity was checked for continuous and categorical variables using STATA multivariate regression spline (MVRS) command. MVRS selects the regression spline model that best predicts the outcome variable. The MVRS indicated a linear spline relationship with humidity. The cutoff value of humidity was determined based on the knots calculated by MVRS and was tested using “mkspline” command in STATA. The mk-spline creates variables that contain a linear spline. These interactions were examined in each season. All analyses were performed using the STATA statistical software version 14 (Stata Corp., College Station, TX, USA). Statistical significance was defined as p < 0.05.
Data availability
The data that support the findings of this study are available from JROAD. However, restrictions apply to the availability of these data, which were used under the approval of the current study and are thus not publicly available. The data are available from JROAD upon reasonable request ([email protected]). Environmental data were obtained from the National Institute for Environmental Studies, Japan (http://www.nies.go.jp/db/index-e.html), and the Japan Meteorological Agency (https://www.data.jma.go.jp/obd/stats/etrn/index.php).
References
-
O’Donnell, M. J. et al. Global and regional effects of potentially modifiable risk factors associated with acute stroke in 32 countries (INTERSTROKE): A case-control study. Lancet 388, 761–775 (2016).
Google Scholar
-
Lo Coco, D., Lopez, G. & Corrao, S. Cognitive impairment and stroke in elderly patients. Vasc. Health Risk Manag. 12(105), 116 (2016).
-
Ministry of Health, Labour and Welfare. Estimates of national medical care expenditure. https://www.mhlw.go.jp/toukei/list/37-21.html.
-
Myint, P. K., Vowler, S. L., Woodhouse, P. R., Redmayne, O. & Fulcher, R. A. Winter excess in hospital admissions, in-patient mortality and length of acute hospital stay in stroke: A hospital database study over six seasonal years in Norfolk, UK. Neuroepidemiology 28, 79–85 (2007).
Google Scholar
-
Hong, Y. C. et al. Ischemic stroke associated with decrease in temperature. Epidemiology 14, 473–478 (2003).
Google Scholar
-
Yoneyama, K. et al. Weather temperature and the incidence of hospitalization for cardiovascular diseases in an aging society. Sci. Rep. 11, 10863 (2021).
Google Scholar
-
Higuma, T. et al. Effects of temperature and humidity on acute myocardial infarction hospitalization in a super-aging society. Sci. Rep. 11, 22832 (2021).
Google Scholar
-
Kleindorfer, D. O. et al. 2021 guideline for the prevention of stroke in patients with stroke and transient ischemic attack: A Guideline from the American Heart Association/American Stroke Association. Stroke 52, e364–e467 (2021).
Google Scholar
-
Tsao, C. W. et al. Heart disease and stroke statistics-2023 update: A report from the American Heart Association. Circulation 147, e93–e621 (2023).
Google Scholar
-
Carroll, D. et al. Blood pressure reactions to the cold pressor test and the prediction of ischaemic heart disease: Data from the Caerphilly Study. J. Epidemiol. Commun. Health 52, 528–529 (1998).
Google Scholar
-
Naraghi, R. et al. Posterior fossa neurovascular anomalies in essential hypertension. Lancet 344, 1466–1470 (1994).
Google Scholar
-
Morimoto, S. et al. Neurovascular compression of the rostral ventrolateral medulla related to essential hypertension. Hypertension 30, 77–82 (1997).
Google Scholar
-
Aoki, S. et al. Blood pressure variability and prognosis in acute ischemic stroke with vascular compression on the rostral ventrolateral medulla (RVLM). Hypertens. Res. 34, 617–622 (2011).
Google Scholar
-
Cao, Y. et al. Air pressure, humidity and stroke occurrence: A systematic review and meta-analysis. Int. J. Environ. Res. Public Health 13, 675 (2016).
Google Scholar
-
Slatina, E. et al. Correlation between change in air humidity and the incidence of stroke. Mater. Sociomed 25, 242–245 (2013).
Google Scholar
-
Lockett, L. J. Hydration-dehydration, heat, humidity, and “cool, clear, water”. Sports Med. Arthrosc. 20, 240–243 (2012).
Google Scholar
-
Keatinge, W. R. et al. Increases in platelet and red cell counts, blood viscosity, and arterial pressure during mild surface cooling: Factors in mortality from coronary and cerebral thrombosis in winter. Br. Med. J. (Clin. Res. Ed.) 289, 1405–1408 (1984).
Google Scholar
-
Woodhouse, P. R., Khaw, K. T., Plummer, M., Foley, A. & Meade, T. W. Seasonal variations of plasma fibrinogen and factor VII activity in the elderly: Winter infections and death from cardiovascular disease. Lancet 343, 435–439 (1994).
Google Scholar
-
Portegies, M. L., Koudstaal, P. J. & Ikram, M. A. Cerebrovascular disease. Handb. Clin. Neurol. 138, 239–261 (2016).
Google Scholar
-
Matongo, T. J., Tamba, J. G., Mba, L. & Yamb, E. Experimental data showing the thermal behaviour of a residential building in a hot and humid climate on three scenarios: An empty room with a closed door, an empty room with an open door, and a normal inhabited room. Data Brief 41, 107906 (2022).
Google Scholar
-
Yasuda, S. et al. The current status of cardiovascular medicine in Japan—Analysis of a large number of health records from a nationwide claim-based database. JROAD-DPC. Circ. J. 80, 2327–2335 (2016).
Google Scholar
-
Yasunaga, H., Ide, H., Imamura, T. & Ohe, K. Impact of the Japanese diagnosis procedure combination-based Payment System on cardiovascular medicine-related costs. Int. Heart J. 46, 855–866 (2005).
Google Scholar
-
Nakamura, K. Diagnosis procedure combination database would develop nationwide clinical research in Japan. Circ. J. 80, 2289–2290 (2016).
Google Scholar
-
Yasunaga, H., Matsui, H., Horiguchi, H., Fushimi, K. & Matsuda, S. Application of the diagnosis procedure combination (DPC) data to clinical studies. J. UOEH 36, 191–197 (2014).
Google Scholar
-
Charlson, M. E., Pompei, P., Ales, K. L. & MacKenzie, C. R. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J. Chron. Dis. 40, 373–383 (1987).
Google Scholar
Acknowledgements
We would like to thank T. Higuma for useful discussions. We would like to thank Editage (wwww.editage.com) for English language editing.
Funding
This work was supported by the Japan Society for the Promotion of Science (KAKENHI Grant Number JP21K08118) and Pfizer Health Research Foundation (Grant Number 21-Y-01).
Author information
Authors and Affiliations
Contributions
S.D and K.Y contributed equally to this work. S.D, K.Y., T.Y., and Y.K. had full access to all the data in the study and take responsibility for the integrity of the data and accuracy of the data analysis. Concept and design: All authors. Acquisition, analysis, or interpretation of data: Y.I., M.I., and Y.T. Drafting of the manuscript: S.D, K.Y., Y.K, and M.N. Critical revision of the manuscript for important intellectual content: All authors. Statistical analysis: S.D., K.Y., and M.N. Administrative, technical, or material support: M.N and Y.S. Supervision: Y.I, M.I., Y.T, T.H., and Y.J.A.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
Reprints and Permissions
About this article
Cite this article
Doi, S., Yoneyama, K., Yoshida, T. et al. Effects of temperature and humidity on cerebrovascular disease hospitalization in a super-aging society.
Sci Rep 13, 20602 (2023). https://doi.org/10.1038/s41598-023-47998-6
-
Received: 23 May 2023
-
Accepted: 21 November 2023
-
Published: 23 November 2023
-
DOI: https://doi.org/10.1038/s41598-023-47998-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.