Infection
Cheap fungal nail infection drug found to work on crippling flesh-eating disease
A cheap and easily taken drug used to treat fungal nail infections has been found to be highly effective against a devastating flesh and bone-eating disease found across Africa, Asia and the Americas.
Researchers say the breakthrough offers hope to thousands of patients who have suffered decades of neglect and can face amputations if the disease is left untreated.
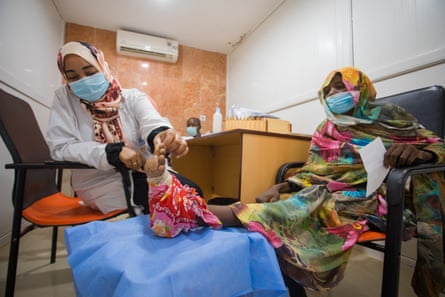
Results of the first clinical trial for a new treatment for mycetoma, which took place in Sudan, have shown that the oral drug fosravuconazole is up to 85% effective and has no side-effects.
Dr Borna Nyaoke, the head of mycetoma at the Drugs for Neglected Diseases Initiative (DNDi) – which coordinated the trial with the Mycetoma Research Center (MRC) in Sudan’s capital, Khartoum, and the Japanese pharmaceutical company Eisai – labelled the discovery “momentous”.
“We were all very excited,” she said. “It’s going to be a gamechanger.”
Mycetoma is a chronic infection caused by certain bacteria and fungi and is on the World Health Organization’s list of 20 neglected tropical diseases. There are no accurate figures on the global number of cases, but Sudan and Mexico report the highest number.
The condition primarily affects young adults in poorer, rural areas, with children making up about 20-25% of all mycetoma patients. Many people become infected through thorn pricks while walking barefoot.
If left untreated, it can lead to amputation and death. The stigma associated with the disease can have serious mental health consequences.
At present, mycetoma is treated with the drug itraconazole, which needs to be taken with food four times a day for a year and has a number of side effects. It is also expensive, costing about $2,000 (£1,600) to treat a patient for a year. It is not available in all endemic countries.
Fosravuconazole, which is already used to treat fungal nail infections, can be taken once a week for a year without food. “In our population, where having two square meals a day is difficult, this is an advantage,” said Nyaoke, who added that the new treatment is expected to be much cheaper.
However, despite the successful trials in Sudan, the war in the country has derailed efforts to fight the disease. Sudan was plunged into a violent crisis in April after fighting broke out in April between the Sudanese armed forces, led by Gen Abdel Fattah al-Burhan, and the Rapid Support Forces loyal to his rival Mohamed Hamdan Dagalo, known as Hemedti.
The MRC in Khartoum, the only dedicated centre for mycetoma in the world and which acts as a reference laboratory for many medical facilities, has been forced to close.

Nyaoke said Sudan’s regulatory authority appeared ready to approve the drug for nationwide use, but it is unknown when a decision will be made.
Ahmed Fahal, a professor of surgery at Khartoum University and founder of the centre, said: “The war had a lot of impact in Sudan in general as well as on the mycetoma centre and on patients. It affected our work; staff have fled and are traumatised. The war has definitely moved us backwards.”
Fahal is hoping to open two new centres in areas of the country not affected by conflict. “We are determined to start again and continue what we are doing,” he said.