Infection
What you need to know about COVID and vaccines now
NSW is in the grip of its eighth COVID-19 wave with the latest NSW Health data revealing that infection rates have doubled since the beginning of October.
Released on Thursday, the data shows a 16 per cent increase in community infections in the fortnight to November 18, compared with the previous reporting period, for those who have tested positive for COVID-19 via PCR testing.
Since the beginning of October, COVID-19 numbers have increased by 94 per cent. NSW Health now releases its epidemiological report only fortnightly.
The total number of people presenting at emergency departments in the past fortnight was also higher than for the previous two weeks, NSW Health said, however, the proportion who required admission remained stable, indicating that the severity of the virus circulating in the community remained unchanged.
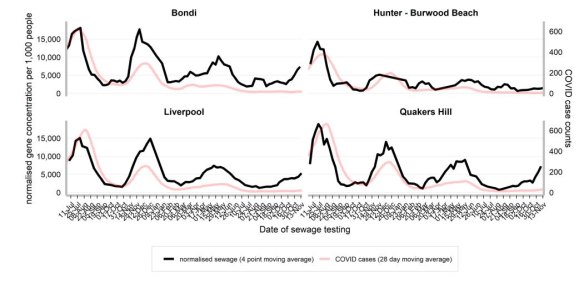
Self-reporting of COVID-19 positive RAT results ceased at the end of September, with authorities turning to the NSW Sewerage Surveillance Program to test fragments on SARS-CoV-2, to gain an insight into virus spread. The latest data indicates the concentration of COVID-19 in Bondi, Liverpool and Quakers Hill is higher than in other parts of Sydney, and rising.
Rising rates of COVID detected in Sydney’s wastewater (black line).Credit: NSW Health
The dominant variants
The variant EG.5 now accounts for more than half of all variants in the state, however, the proportion of samples in which BA.2.86 has been detected is increasing.
The nation’s emergency response to the COVID-19 pandemic formally ended on October 20, with the country’s chief health officer declaring that there was no longer a need for the virus to be considered a Communicable Disease Incident of National Significance.
At the beginning of the month, Kelly told this masthead that Australia was well protected by immunity built up through vaccination and infections and could move to a “business as usual” response to COVID.
The new booster
With that came news that the new monovalent vaccines would be available to eligible Australians from December 1, following approval by the Australian Technical Advisory Group on Immunisation (ATAGI). The new XBB.1.5. vaccines target a sub-variant of the Omicron strain and have been approved for use as both primary and additional doses, with Pfizer’s version approved for eligible people aged over five years, and Moderna’s for those over 12.
The vaccines offer modestly improved protection against COVID-19 strains circulating in Australia, according to a federal government statement.
Vaccine eligibility and advice
Kirby Institutes’s Associate Professor Stuart Turville.Credit: James Brickwood
University of NSW Associate Professor Stuart Turville, who works out of the Kirby Institute, said that while the new vaccines were not specific to EG5, EG5 and XBB.1.5. were “very closely related” and the vaccines would offer significant benefits.
“A dynamic of our immune system is that it is often better the longer you leave it [to get a booster]. There have been studies done that have shown if you have a really short distance between your booster, it offers the best protection,” he explained.
“A lot of people naively think I need to have a booster every three months, or I need to have (a booster at the) distance that is available to me because they think that keeping the antibodies up is the best thing.
“But what protects you the best is that you give your body a break, and what that does is it cools the jets a bit, and then your immune cells actually beautifully mature over that time, and then you are producing quality rather than quantity, and the antibodies left actually do a really, really good job.”
ATAGI does not, at this stage, recommend a second 2023 booster for healthy people under 65.
Turville said that ATAGI’s vaccine advice was “fluid” and encouraged individuals to monitor for any changes.
He added that while COVID-19 may no longer be “front page news”, there was a lot of scientific activity in the background surveilling what the virus was doing.
“People need to be confident that there are really smart measures that we have learnt over the last three years that are consolidated, and they’re very sharp and purposeful, to feedback to us so we understand what the virus is doing,” Turville said.
Antiviral eligibility
Oral antiviral treatments Lagevrio and Paxlovid remain approved for people who are vulnerable to severe disease as they can help slow COVID-19 infections.
The federal government recommends that those eligible start antivirals as soon as possible after symptoms begin. The advice has not been updated since July.
Those who may be eligible for PBS-subsidised antiviral medication are people over 70 years of age, those who are 50 and who have a comorbidity, as well as First Nations people over 30 who have one additional risk factor for developing severe disease.
People who are severely immunocompromised, and who are over 18, are also eligible for antiviral medication. These include people who have blood cancer, who are a transplant recipient, who have undergone chemotherapy or radiotherapy in the past three months, who have congenital heart disease or who have cerebral palsy or Down Syndrome.
The Morning Edition newsletter is our guide to the day’s most important and interesting stories, analysis and insights. Sign up here.