Infection
Cryptosporidium infections in asymptomatic calves up to 4 months in Poland: a cross-sectional population study
Abstract
Cattle cryptosporidiosis is noted worldwide with varied frequency of infection prevalence depending on geographical, environmental and husbandry factors. In this study, the prevalence of Cryptosporidium infections in cattle was determined on the basis of molecular results obtained by testing 1601 faecal samples collected from calves up to 4 months of age housed in all Polish provinces from 2014 to 2018. Detection and identification of Cryptosporidium species was performed at the 18 small subunit ribosomal RNA (18S rRNA) locus by conducting PCR–RFLP analysis of the amplified DNA fragments. The prevalence of Cryptosporidium infections in the cattle population was 45.3% (CI 95%: 42.8–47.7; 725/1601). The infected animals were housed on 233/267 (87.3%) of monitored farms with regional prevalence ranging from 27.8 to 62%. The restriction pattern of 18S rRNA amplicons for positive samples was characteristic of C. parvum, C. bovis, C. ryanae, C. andersoni, and unexpectedly also of C. baileyi and C. suis. Infections of C. bovis and C. ryanae prevailed in the studied cattle population relegating C. parvum to third in prevalence. Likewise, mixed infections caused by C. bovis and C. ryanae as well as C. parvum and C. bovis were observed. A relationship between the infecting parasite species and animal breed was found. For instance, C. parvum prevailed in Black and White lowland breed, C. ryanae in Limousine cattle and C. andersoni in dairy animals of mixed dairy breeds. Furthermore, differences in prevalence of particular parasite species between cattle breeds were also shown.
Introduction
Cryptosporidium infections in cattle were recognised for the first time in 1971 in the United States of America in an 8-month-old diarrhoeic female calf. Histopathology of the small intestine revealed atrophy of the villi and the presence of various developmental forms of Cryptosporidium in the epithelium1. Since then, Cryptosporidium infections and cryptosporidiosis have been reported in cattle worldwide2. The significant role of cattle as a source of Cryptosporidium for humans was recognised during a waterborne epidemic of human cryptosporidiosis in Milwaukee, USA3. Likewise in Poland, studies on occurrences of Cryptosporidium in bovine host have been conducted since the 1970s, usually being confined to animals bred on farms from selected Polish regions4,5,6 and using methods not always conducive to identification of the parasite species4,7,8. However, an attempt to assess the prevalence of infections nationwide by individual Cryptosporidium species in Polish cattle of different ages has also been undertaken9. As a pilot study, it covered a broad group of animals at the age from 1 day to 6 years of different health status. However, due to the low number of samples obtained from particular Polish provinces, the results did not allow for a regional assessment of the infection prevalence as well as to gather unbiased data on the occurrence of Cryptosporidium species in the population. It has been shown that infections caused by different Cryptosporidium species mainly affect animals up to the age of 4 months with Cryptosporidium bovis (C. bovis) as predominating species.
A wider application of molecular tools in veterinary parasitology has facilitated identification of Cryptosporidium species in livestock. As with other similar studies conducted in Europe, in Poland the infections previously diagnosed in cattle were mostly caused by Cryptosporidium parvum (C. parvum)8,10,11. The tested animals were also positive for C. bovis, Cryptosporidium andersoni (C. andersoni) and Cryptosporidium ryanae (C. ryanae)9,11. An age-related pattern of Cryptosporidium species infecting cattle was solely observed for C. parvum. C. andersoni was the only species occurring in adult cattle over one year of age9. Species which were unusual for the bovine host, such as Cryptosporidium felis (C. felis), were only occasionally detected in cattle populations in Poland12.
In an effort to gather data on the prevalence of Cryptosporidium infections in cattle population and in particular Polish provinces, only healthy animals up to the age of 4 months were sampled. Apart from a molecular assessment of the infection prevalence, the study aimed to identify parasite species and their regional distribution pattern in cattle of different breeds and ages.
Materials and methods
Ethics approval and consent to participate
This study did not require approval of an Ethics Committee. However, freshly voided faeces were collected during routine veterinary practice in adherence to international guidelines for animal care. Sample collection was not harmful and did not violate animal welfare laws. No clinical interventions were performed. The owners of the cattle included in this study provided informed consent to participate.
Cattle faeces
Over a period of 5 years, 1601 freshly voided faecal samples were collected from cattle from the age of 1 week to 4 months from 2014 to 2018 (Table 1). Each cattle was sampled only once and the sampling plan covered each year different provinces. The animals were housed on 267 farms located across the 16 administrative provinces of Poland. The average number of animals in the herd was 137. The cattle were mainly raised on large farms (175, 65.5%) with > 50 heads. Small farms (< 50 heads) accounted for 92 (34.5%) of the tested establishments. In each year from 306 to 324 faeces were subjected for testing. Animals originated from farms located in districts with the highest cattle populations in 3 or 4 provinces each year13. In each province 18 farms were monitored except from Dolnośląskie (DS) (15 farms) and Opolskie (OP), Śląskie (SL) and Podkarpackie (PK) where 12 farms were sampled in each localization. Farms were randomly selected and represented different administrative locations in the province. Generally, from each farm freshly voided faeces of 6 animals (18 per district) of different breeds at the age between 1 week to 4 months were taken. However, there were also farms enrolled on which all sampled animals were the same age and breed. For instance, on 9 farms, there were only 4-week-old calves and on 15 farms (5 with calves of each age) there was stock at the age of 8, 12, and 16 weeks. The cattle were in good health without symptoms of Cryptosporidium infections. During this study no clinical intervention or animal examination were conducted by vets taking care of these farms who collected samples. The animals were divided into three age groups as shown in Table 1. Faecal samples of 10–15 g were placed individually into plastic containers, labelled and sent to the laboratory. Faeces were collected from cattle representing dairy breeds (Polish Black and White Holstein–Friesian (HO), Jersey (JE), Polish Red and White Holstein–Friesian (RW), Brown Swiss (BS), and dairy animals of mixed dairy breeds (MS)); meat breads (meat cattle of mixed meat breeds (MM), Aberdeen Angus (AN), Charolaise (CH), Salers (SL), Limousine (LM), and Belgian Blue (BB)); and cattle of mixed dairy-meat breeds (mixed dairy-meat breeds (MDM), Black and White lowland (NCB), Polish Red (RP), Montbéliarde (MO), Polish Black and White (ZB) and Simmental (SM)). The sampled animals mainly represented dairy breeds (74.8%), followed by meat (14.5%) and mixed (10.7%) breeds.
Detection and identification of Cryptosporidium species based on the analysis of the 18S rRNA gene fragment
Cryptosporidium DNA was extracted from 0.1 g (100 µl) of the faeces with an alkali wash and a heat lysis method developed by Millar et al.14 with further modifications9. Nucleic acid extracts were purified using a GeneMATRIX PCR/DNA Clean-Up Purification Kit (EURx Ltd., Gdańsk, Poland) according to the manufacturer’s instructions. The extracts containing parasite DNA were stored at − 20 °C until use. Detection of Cryptosporidium DNA was performed by a nested-PCR method allowing amplification of the 18S rRNA Cryptosporidium gene fragment using the primers specified by Xiao et al.15 and conditions described elsewhere16. To increase amplification efficiency and to reduce amplification inhibition each reaction mixture was supplemented with 20 µg of bovine serum albumin (Thermo Fisher Scientific, Vilnius, Lithuania)9. The correct performance of the nucleic acid extraction and the amplification step were verified by inclusion of an appropriate set of controls as described elsewhere9. All reactions were performed in a Biometra TProfessional BASIC thermocycler (Analytik Jena, Jena, Germany).
The identity of Cryptosporidium species in positive faeces was defined by a PCR–RFLP analysis of the amplified 18S rRNA fragments. The analysis used the following enzymes: NdeI for identification of C. parvum17, MboII in a parallel digestion for C. bovis and Cryptosporidium ryanae (C. ryanae) detection18, XbaI for differentiation of C. andersoni and C. parvum by their lack of a restriction site from C. bovis and C. ryanae9, BcuI (SpeI) in digestion for identification of Cryptosporidium suis (C. suis)17 and SspI for detection of Cryptosporidium baileyi (C. baileyi)19. To confirm the correct identification of Cryptosporidium species which were unusual for cattle such as C. suis and C. baileyi, sequencing of the post-PCR 18S rRNA amplicons was conducted. The PCR products excised from the agarose gel and purified were directly sequenced in both directions using the ABI Prism BigDye Terminator v3.1 Cycle Sequencing Kit on an ABI 3730XL DNA sequencer (Life Technologies, Carlsbad, CA, USA) at the Genomed S.A. sequencing service (Warsaw, Poland). Nucleotide sequences were aligned with published sequences from GenBank by using the NCBI-BLAST program (http://blast.ncbi.nlm.nih.gov/Blast.cgi). The 18S rRNA nucleotide sequences of C. baileyi and C. suis were deposited in GenBank under the accession numbers OP090504-OP090507.
Statistical analyses
The prevalence of Cryptosporidium infections in cattle in particular Polish provinces was estimated by the Clopper-Pearson method. It was also used to assess prevalence of infections in age groups of animals as well as age-related prevalence of detected parasite species. Subsequently, a chi-squared (χ2) test with Yates’ continuity correction was employed to assess infection prevalence between age groups of animals and to determine dominating Cryptosporidium species in cattle. Concluding the statistical work, the uncorrected χ2 test and odds ratio in logistic regression was employed to analyse differences in regional infection prevalence in animals from different age groups, prevalence of Cryptosporidium species between provinces and animal breeds. The calculations were performed using R software v. 4.1.120 with “prevalence”21 and “epiR”22 packages.
Results
Detection of Cryptosporidium infections in cattle
The 18S rRNA gene fragment was successfully amplified in 725 out of 1601 cattle faecal samples (Table 2). The restriction pattern of 18S rRNA amplicons characteristic for C. parvum, C. bovis, C. ryanae, and C. andersoni was shown for 100, 368, 211, and 54 DNA samples respectively. Digestion by SSpI revealed the presence of C. baileyi in two samples. Two samples were also positive for C. suis when treated with SSpI and BcuI (SpeI). Twelve samples contained mixtures of two different sequences such as C. parvum and C. bovis and C. bovis and C. ryanae. Sequence analysis of the 18S rRNA fragments of C. baileyi and C. suis confirmed the species identity.
Geographical distribution of Cryptosporidium infections
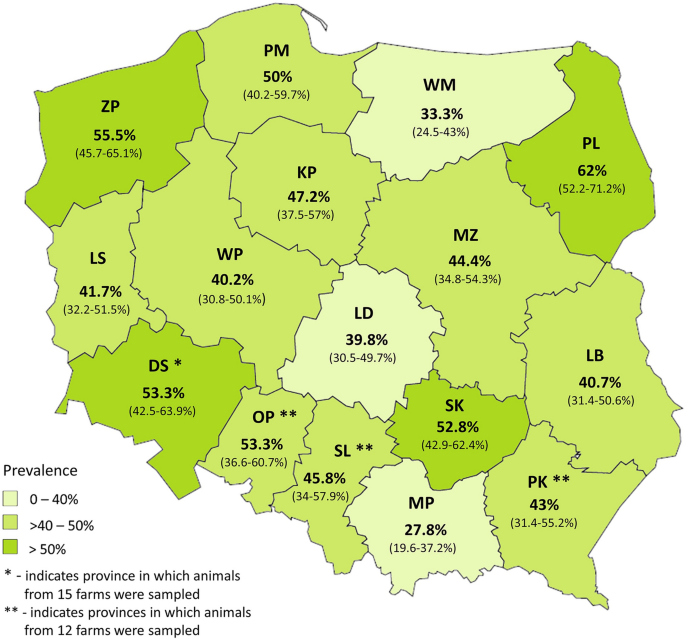
The infected animals were reared on 233 (87.3%) out of 267 monitored farms located across all 16 Polish provinces. In the cases of the Zachodniopomorskie (ZP) and DS provinces, all monitored farms appeared Cryptosporidium positive. Cryptosporidium infections were detected with varied prevalences ranging from 27.8% in Małopolskie (MP) to 62% in Podlaskie (PL) province (Fig. 1). However, greater than 40% prevalence of infections was revealed in the majority of the monitored regions.
Province-related prevalence of Cryptosporidium infections in cattle. The numbers within parentheses indicate 95% CI.
A relationship between the prevalence of Cryptosporidium infections and the age groups of cattle farmed in particular provinces was shown. In calves at 1–4 weeks of age, the highest number of infections was detected in Kujawsko-Pomorskie (KP) and Świętokrzyskie (SK) compared to the following provinces: DS (χ2 = 6.4; p = 0.012; OR 6.2 and χ2 = 5.0; p = 0.025; OR 4.6), Lubelskie (LB) (χ2 = 5.4; p = 0.02; OR 3.2 and χ2 = 4.0; p = 0.046; OR 2.4), Warmińsko-Mazurskie (WM) (χ2 = 5,4; p = 0.02; OR 3.4 and χ2 = 4,0; p = 0.046; OR 2.5) and MP (χ2 = 11.9; p < 0.001; OR 13.6 and χ2 = 10.4; p = 0.001; OR 10.1). Significant differences in prevalence of infections were also seen between DS and Mazowieckie (MZ) (χ2 = 4.1; p = 0.043; OR 0.25) and between all provinces except DS, LB, WM, SL, and PK relative to MP (χ2 = from 11.9 to 4.7; p ≤ 0.03; OR from 13.6 to 5.3). High percentages of infected animals at the age of > 4–8 weeks were found in PL compared to KP (χ2 = 5.8; p = 0.016; OR 3.21), LS (χ2 = 8.0; p = 0.005; OR 4.82), Łódzkie (LD) (χ2 = 6.8; p = 0.009; OR 4.0), Wielkopolskie (WP) (χ2 = 5.4; p = 0.02; OR 3.7), and MP (χ2 = 7.6; p = 0.006; OR = 4.2). They were also observed between DS and Lubuskie (LS) (χ2 = 4.0; p = 0.046; OR 3.1), OP and MP (χ2 = 4.0; p = 0.045; OR 3.3), LS and WM (χ2 = 3.9; p = 0.048; OR 0.3), LS and OP (χ2 = 4.5; p = 0.035; OR 0.3). Generally, Cryptosporidium infections in the oldest group of calves predominated in PL (χ2 = 6.1; p = 0.013; OR 0.5) comparing to other provinces except for SK, Pomorskie (PM), KP, and DS. Likewise, animals at that age were more frequently infected in ZP than in other Polish provinces (χ2 = 25.4; p < 0.001; OR 0.06). Significant differences were also found between the following provinces DS and MZ (χ2 = 6.3; p = 0.012; OR 3.3 ), LD (χ2 = 4.2; p = 0.041; OR 2.4), WM (χ2 = 14; p < 0.001; OR 5.2), PK (χ2 = 6.5; p = 0.011; OR 3.3), and MP (χ2 = 9.3; p = 0.002; OR 3.4) as well as between WP and WM (χ2 = 4.5; p = 0.033; OR 2.5), WM and SL (χ2 = 6.7; p = 0.009; OR 0.28).
Age related prevalence of Cryptosporidium spp.
Overall Cryptosporidium infection prevalence for the studied cattle population was 45.3% (CI 95%: 42.8–47.7). Parasites were detected in all age groups of animals with the following group prevalence: 45.3% (CI 95%: 41–49.7; 1–4 weeks), 48.9% (CI 95%: 44.3–53.6; 4–8 weeks), and 42.4% (CI 95%: 38.5–46.5; 8–16 weeks) (Table 3).
However, statistically significant differences were solely observed between animals at the age of 4–8 and 8–16 weeks (χ2 = 4.19; p = 0.041). Six different species were detected being C. bovis the most common in the studied cattle population (χ2 = 189.13, p < 0.001) (Table 3).
Prevalence of C. bovis in the youngest animals, 49.8% (CI 95%: 43.3–56.3) was similar to that in those above 8 weeks of age, 50.9% (CI 95%: 44.7–57.2). This was also the case with C. ryanae, 24.2% (CI 95%: 18.9–30.2) and 33.2% (CI 95%: 27.4–39.3) , respectively. C. bovis and C. ryanae were the most common species in calves > 8 weeks of age. The overall prevalence of C. parvum in cattle from Poland up to 16 weeks of age was estimated at 6.2% (100/1601). In contrast to infections with C. parvum, the number of infections with C. andersoni increased with an animal age. C. baileyi, C. suis and mixed infections caused by the species pairs of C. bovis and C. ryanae or C. parvum and C. bovis were found in cattle only occasionally (Table 3).
Geographical prevalence of Cryptosporidium species in cattle
There were also differences observed in the prevalence of infections caused by particular parasite species between provinces (Table 4). C. bovis predominated in LS, PM and ZP compared to PK (χ2 = 6.6–2.4; p = 0.01–0.028; OR 2.4–2.7), LD (χ2 = 11.0–0.13; p = 0.001–0.004; OR 3.2–2.8), and WM (χ2 = 5.8–3.8; p = 0.016–0.05; OR 2.2–1.9). Significant differences were also seen between SL and DS (χ2 = 5.1, p = 0.024; OR 0.4), OP and DS (χ2 = 6.1; p = 0.013; OR = 0.4), OP and SK (χ2 = 4.5; p = 0.033; OR = 0.5); PK and SK (χ2 = 7.9; p = 0.005; OR 0.3), PK and KP (χ2 = 4.3; p = 0.039; OR 0.4), LD and PL (χ2 = 3.9; p = 0.047; OR 0.5), LD and KP (χ2 = 7.6; p = 0.006; OR 0.4). Furthermore, C. bovis infections predominated in DS (χ2 = 15.5–4.3; p = 0.001–0.038; OR 4.0–1.9) when compared to other provinces except for SK and KP. Likewise, differences in Cryptosporidium prevalence were found between the following regions SK and LD (χ2 = 13.0, p ≤ 0.001; OR 3.5), MP (χ2 = 5.5; p = 0.019; OR 2.1), LB (χ2 = 4.7; p = 0.03; OR 2.0), MZ (χ2 = 4.7; p = 0.03; OR 2.0), and WM (χ2 = 7.2; p = 0.007; OR 2.4. Except for MP and WM, C. ryanae ranked first in PM than in other Polish provinces (χ2 = 26.2–5.7; p = 0.001–0.017; OR 0.05–0.2). It also prevailed in ZP, SL, OP, PK, SK compared to MP (χ2 = 7.0–3.9; p = 0.008–0.047; OR 3.5–2.5). Other significant differences in prevalence of infections caused by this parasite species were observed between LS and PM (χ2 = 8.7; p = 0.003; OR 7.2), LS and PL (χ2 = 6.8; p = 0.009; OR 0.4), ZP and PL (χ2 = 4.1; p = 0.043; OR 0.5), OP and WM (χ2 = 5.8; p = 0.016; OR 3.0), DS and PL (χ2 = 4.8; p = 0.028; OR 0.4), SK and WM (χ2 = 4.4; p = 0.037; OR 2.5), LD and PL (χ2 = 5.8; p = 0.016; OR 0.4), MP and LB (χ2 = 3.9; p = 0.047; OR 0.4), MP and PL (χ2 = 15.0; p ≤ 0.001; OR 0.2), MP and MZ (χ2 = 4.7; p = 0.03; OR 0.4), LB and PL (χ2 = 4.1, p = 0.043; OR 0.5), PL and KP (χ2 = 7.8; p = 0.005; OR 2.8), PL and WP (χ2 = 10.1; p = 0.001; OR 3.4), PL and WM (χ2 = 13.3; p ≤ 0.001; OR 4.4).
C. parvum occurred with significantly higher prevalences in PM (χ2 = 14.1–6.6, p = 0.001–0.01; OR = 3.4–17.8) relative to the remaining provinces with the exception of ZP, PK, LD, and MZ. Likewise, its dominance was noted in ZP compared to SL, OP, DS, SK, MP, LB, and WM (χ2 = 9.3–3.9; p = 0.002–0.049; OR 2.8–12.2). Regional differences in the number of C. parvum isolations were found between LS, SL, OP, DS and PK (χ2 = 6.5–3.9; p = 0.011–0.049; OR 0.1–0.2) as well as between LS, SL, OP, DS, SK and LD (χ2 = 9.3–4.8; p = 0.002–0.028; OR 0.1–0.2). They were also found for LS and PM (χ2 = 11.9; p ≤ 0.001; OR 0.1), LS and ZP (χ2 = 6.7; p = 0.009; OR 0.2), PK and MP (χ2 = 5.4; p = 0.02; OR 6.6), LD and MP (χ2 = 8.7; p = 0.003; OR 7.2), LD and LB (χ2 = 3.9; p = 0.049; OR 2.8), LS and MZ (χ2 = 4.0; p = 0.045; OR 0.3), DS and MZ (χ2 = 6.2, p = 0.013; OR 0.1), SL and MZ (χ2 = 5.4, p = 0.02; OR value not estimated due to a low sample size), SK and MZ (χ2 = 4.0; p = 0.045; OR 0.3), MP and MZ (χ2 = 5.6; p = 0.017; OR 0.2), LD and WM (χ2 = 5.2; p = 0.023; OR 3.6), PK and SK (χ2 = 3.9; p = 0.049; OR 4.4).
The provinces at the opposite extreme were SL for C. parvum and LS, ZP and MZ for C. andersoni, where these parasites were not detected. Isolations of C. suis and C. baileyi from cattle were achieved in PL and WP and in LS and SL respectively (Table 4). Mixed infections of C. bovis and C. ryanae with a 2.8% frequency were observed in WP and below 1.8% in LS, ZP, SL, OP and DS.
Breed–related prevalence of Cryptosporidium spp.
Besides BB and SL animals other breeds were found positive for Cryptosporidium DNA. The majority of infections (71.7%; 520/725) were detected in dairy cattle of HO breed (Table 5). Cryptosporidium prevalences were also high in MM (8.3%; 60/725) and SM (5.2%; 38/725) breeds. Analysing occurrence of infections within the breed, C. parvum prevailed in NCB (χ2 = 442; p < 0.001; OR 38.3), C. ryanae in LM (χ2 = 10.3; p = 0.01; OR value not estimated due to low sample size) and C. andersoni in MS cattle (χ2 = 103; p > 0.01; OR 4.6). It is noteworthy that also differences in prevalence of parasite species between some breeds were observed. For instance, C. bovis was significantly more often detected in HO compared to LM (χ2 = 3.8; p = 0.05). This relation was also seen between other breeds such as LM and SM (χ2 = 5.7; p < 0.05) as well as for LM and RW (χ2 = 4.3; p < 0.05). Likewise, C. bovis predominated in SM (χ2 = 5.2; p < 0.05) and RW (χ2 = 5.5; p < 0.05) cattle compared to animals of MS breed. C. ryanae infections prevailed respectively in HO (χ2 = 11.5; p < 0.01) and LM (χ2 = 12.8; p < 0.01) breeds relative to LM and SM cattle.
Discussion
Molecular methods have become irreplaceable in the detection and identification of Cryptosporidium species. PCR–based methods are gradually replacing microscopic methods in epidemiological studies on Cryptosporidium prevalence in livestock23,24,25,26,27,28,29 as they offer higher sensitivity and allow precise and direct identification of parasite species30,31. In this study, the prevalence of Cryptosporidium infections in cattle was only determined on the basis of molecular testing followed by the identification of parasite species using RFLP analysis. It was fast and reliable alternative to amplicon sequencing which often fails for samples containing a mixture of closely related parasite strains or species. To our knowledge this is the first nationwide epidemiological study on Cryptosporidium prevalence conducted in Europe, although a similar type of research has already been performed in China31. In contrast to our work, other Cryptosporidium studies carried out in cattle in Europe were only limited to animal populations kept regionally in each country32,33,34,35,36,37.
The prevalence of Cryptosporidium infections in cattle from Poland at the age of 1 to 16 weeks was 45.3%, with only significant difference in parasite prevalence between the older animal groups. Nevertheless, regional differences in prevalence of infections related to animals age were observed. In the north–western, central and eastern regions of Poland, a higher number of infections was found in the youngest animals up to 8 weeks of age. This can to some extend be explained by the presence of a higher cattle population housed in these regions with majority of animals represented by dairy breeds. There is a marked difference from the prevalence found in our previous study aiming at detection and molecular identification of Cryptosporidium species in cattle between the ages of 1 day and 6 years, in which it was on average 17%9. This large discrepancy could have resulted from testing of approximately threefold higher number of animals which gave more accurate data on Cryptosporidium prevalence at province level as well as from different sampling plan used in the current study. The narrow age range (1–16 weeks) of the sampled cattle may have also contributed to the results obtained as in older animals which were not tested in this study, Cryptosporidium infections occur less frequently38,39. It should be emphasised that sampled animals originated from randomly selected farms of different sizes which were located in the districts of their provinces with the highest cattle populations. Therefore, it is likely that higher density of animal population in the studied regions could have facilitated infection spread between animals and farms. This observation has previously been shown on commercial cattle farms40,41,42. Likewise, an association between dairy herd size and an increased risk of Cryptosporidium infection has been demonstrated43. Also shown in this study, the intensive cattle rearing system was mainly associated with dairy farming, which might have influence on prevalence of Cryptosporidium infection, as in all provinces with infection rate above 50%, dairy farming was the main rearing system. A direct comparison of the results obtained in this study with those of other European studies is difficult, as most of them focused on small animal populations and a discrete region25,42 or were limited to selected farms44. Certainly, the higher Cryptosporidium prevalence of the present study cannot be attributed to differences in sample processing and detection methodology, because those studies also employed molecular methods. As an outcome comparable to our results, a high 48.6% rate of Cryptosporidium prevalence was observed in cattle in a cohort study conducted in Sweden45 and 43.8% prevalence in dairy cattle herds in Cyprus46. Contrastingly to the European data, a low 14.1% infection rate was recorded in a large population study on pre–weaned cattle under the age of 3 months from northwest China47. Surprisingly, a low 9.9% infection prevalence was estimated for diarrhoeic calves in Korea48. Differences in infection prevalence can be found despite age similarity of the tested animals and size similarity of the sampled animal population. Various factors such as geographical location, environmental conditions, animal breed, husbandry system and rearing conditions may have an influence on the results obtained.
In the current study the majority of infections in calves up to 4 months were caused by C. bovis and C. ryanae. The species distribution in animals has changed in Poland over time9 as currently C. parvum infections were reported less frequently. A similar infection pattern of detected species with dominating C. bovis and C. ryanae has also been shown in several studies from different parts of the world45,49. As demonstrated here, C. parvum was not the dominant species in healthy calves up to 8 weeks of age, and this finding is in agreement with previous observations45. Consequently, it is not surprising that different distribution patterns of C. parvum have been found depending on the health status of the tested animals33,50,51. In this study, the overall prevalence of C. parvum in cattle in Poland was estimated at 6.2%. It was also found that animals under the age of 1 month were not the major host for C. parvum , and the number of C. parvum–positive samples decreased with a higher animal age. These results are consistent with our previous observations on C. parvum prevalence in cattle in Poland9. Among the Cryptosporidium species identified in this study, C. parvum is considered the most important zoonotic species.
However, in this study unusual Cryptosporidium species for a bovine host, namely C. baileyi and C. suis, were detected in the herds investigated, in which infected animals were carrying those Cryptosporidium species asymptomatically. The calves positive for C. baileyi and C. suis represented different breeds (LM, HO and NCB) as well as rearing types (meat, dairy and dairy–meat varieties). Thus far, C. baileyi52, C. suis53,54, Cryptosporidium scrofarum55, Cryptosporidium xiaoi34, Cryptosporidium serpentis, Cryptosporidium tyzzeri56, C. felis57 and Cryptosporidium occultus (previously known as C. suis–like)34,55 have been detected in grazing ruminants without evidence that they were causing disease. In this study, the presence of C. baileyi and C. suis in cattle might be associated with an accidental oocyst ingestion with contaminated feed. By examining the routes whereby C. baileyi could have been transmitted, it seems likely that feed contamination occurred as a result of environmental parasite abundance linked to extensive poultry farming in areas where the calves were being reared. However, if this finding would be reported as repeatable, a further investigation would be required to reveal any possible parasite–host interactions. In the present study, mixed infections of C. bovis and C. ryanae or C. parvum and C. bovis were detected. They were previously found sporadically in young cattle in Poland9, China (C. parvum and C. bovis)29 and Brazil (C. parvum and C. bovis; C. parvum and C. ryanae; C. parvum and C. andersoni)39. Majewska et al.58 also detected concurrent parasite infections in Polish cattle, but in contrast to our findings, they were caused by C. parvum and C. andersoni.
Significant differences in Cryptosporidium prevalence (from 27.8% to 62%) in Polish provinces were observed. Those results are also in agreement with reports from Korea describing a wide range of prevalence rates of infections between regions48. In this study a higher prevalence of Cryptosporidium infections in particular provinces was not always correlated with a higher population of housed animals, for example, in MP province with the lowest 27.8% Cryptosporidium prevalence, the cattle population was similar to this observed in PM with 50% frequency of infections. Of note is that the prevalence above 50% was mostly observed in provinces in which the tested animals were housed on farms with an intensive cattle rearing system which is mainly associated with dairy farming. Likewise, there were differences observed between species distribution and the region of the country. There were also no differences found in the frequency of infections caused by particular Cryptosporidium species in animals representing the intensive cattle rearing system compared to general cattle population.
Little is known about Cryptosporidium occurrence in cattle of different breeds. When data on Cryptosporidium prevalence was broken down by breed type, a relationship between the infecting specific parasite species and the animal breed was found. For instance, C. parvum prevailed in NCB, C. ryanae in LM and C. andersoni in MS cattle. However, the observed in this study prevalence of breed–associated parasite species should be interpreted with caution because of differences in the numbers of tested animals of each production purpose: the herds were of mostly dairy breeds with HO dominating animals and meat breeds were the next largest proportion. In this light, the current results do not necessarily indicate any greater breed sensitivity to Cryptosporidium infection. It seems to be justified that a higher number of animals of a particular breed type be tested to reveal exact parasite–host interactions. However, breed–related prevalence of Cryptosporidium infections has also been demonstrated in a comparison between European–bred animals, Zebu and animals cross–bred with Zebu39. While this relation was not indicated in our previous study related to Cryptosporidium epidemiology in cattle, it was evident for goats which showed breed–related differences in parasite prevalence59. In this study the distribution of Cryptosporidium species at the farm level was not analysed. Considering the randomness of sampling and varied ages of calves sampled on each farm, those farm–level variables would not guarantee obtaining reliable species distribution results. As a final observation of a possible limitation, although our sampling scheme covered all Polish provinces, the number of sampled animals may not reflect the actual population size in each region. Nevertheless, the sampling plan used allowed to estimate the prevalence of Cryptosporidium infections with 95% probability for the results with a binomial distribution. Only prevalences assessed for particular Polish provinces can be characterized by lower probability. However, the large, total sample size allowed to draw valid conclusions. Furthermore, testing of young animals < 4 months of age in which Cryptosporidium infections are mostly prevalent can be considered a sentinel study for occurrence of Cryptosporidium species in the population.
Conclusions
The results of this cross–sectional population study provide evidence that in clinically healthy population of young calves up to 4 months from Poland, C. bovis and C. ryanae infections predominate relegating C. parvum to third in prevalence. Mixed infections caused by C. bovis and C. ryanae as well as C. parvum and C. bovis were also observed. Likewise, detection of the unusual C. baileyi and C. suis in grazing animals was achieved. This is the first report describing their presence in the population of cattle in Poland.
Data availability
The sequences of 18S rRNA fragment gene of C. baileyi and C. suis strains were deposited into the NCBI GenBank under accession numbers OP090504-OP090507.
Abbreviations
18S
rRNA
:-
Small subunit rRNA gene
- AN:
-
Aberdeen Angus
- BB:
-
Belgian Blue
- BS:
-
Brown Swiss
C. andersoni
:-
Cryptosporidium andersoni
C. baileyi
:-
Cryptosporidium baileyi
C. bovis
:-
Cryptosporidium bovis
C. parvum
:-
Cryptosporidium parvum
C. ryanae
:-
Cryptosporidium ryanae
C. suis
:-
Cryptosporidium suis
- CH:
-
Charolaise
- DS:
-
Dolnośląskie (province)
- HO:
-
Polish Black and White Holstein–Friesian
- JE:
-
Jersey
- KP:
-
Kujawsko-Pomorskie (province)
- LB:
-
Lubelskie (province)
- LD:
-
Łódzkie (province)
- LM:
-
Limousine
- LS:
-
Lubuskie (province)
- MDM:
-
Mixed dairy-meat breeds
- MM:
-
Mixed meat breeds
- MO:
-
Montbéliarde
- MP:
-
Małopolskie (province)
- MS:
-
Mixed dairy breeds
- MZ:
-
Mazowieckie (province)
- NCB:
-
Black and White lowland
- OP:
-
Opolskie (province)
- PCR:
-
Polymerase chain reaction
- PK:
-
Podkarpackie (province)
- PL:
-
Podlaskie (province)
- PM:
-
Pomorskie (province)
- RFLP:
-
Restriction fragment length polymorphism
- RP:
-
Polish Red
- RW:
-
Polish Red and White Holstein–Friesian
- SL:
-
Salers
- SL:
-
Śląskie (province)
- SM:
-
Simmental
- SK:
-
Świętokrzyskie (province)
- WM:
-
Warmińsko-Mazurskie (province)
- WP:
-
Wielkopolskie (province)
- ZB:
-
Polish Black and White
- ZP:
-
Zachodniopomorskie (province)
References
-
Panciera, R. J., Thomassen, R. W. & Garner, F. M. Cryptosporidial infection in a calf. Vet. Pathol. 8, 479–484 (1971).
-
De Graaf, D. C., Vanopdenbosch, E., Ortega-Mora, L. M., Abbassi, H. & Peeters, J. E. A review of the importance of cryptosporidiosis in farm animals. Int. J. Parasitol. 29, 1269–1287 (1999).
Google Scholar
-
Mac Kenzie, W. R. et al. A massive outbreak in Milwaukee of cryptosporidium infection transmitted through the public water supply. N. Engl. J. Med. 331, 161–167 (1994).
Google Scholar
-
Kozakiewicz, B. & Maszewska, I. Epizootiological studies on the invasion of Cryptosporidium sp. in cows in the large farm. Med. Weter. 44, 726–728 (1988).
-
Pilarczyk, B., Ramisz, A. & Jastrzebski, G. Internal parasite of cattle in select Western Pomerania farms. Ann. Parasitol. 48, 383–390 (2002).
-
Pilarczyk, B., Balicka-Ramisz, A. & Ramisz, A. Prevalence of Cryptosporidium sp. in calves from cows imported as in- calf heifers from the Netherlands. Med. Weter. 59, 1135–1136 (2003).
-
Majewska, A. C., Werner, A. & Sulima, P. Prevalence of cryptosporidiosis in Wielkopolska region. Ann. Parasitol. 44, 471 (1998).
-
Bednarska, M., Bajer, A. & Siński, E. Calves as a potential reservoir of Cryptosporidium parvum and Giardia spp. Ann. Agric. Environ. Med. 5, 135–138 (1998).
Google Scholar
-
Rzeżutka, A. & Kaupke, A. Occurrence and molecular identification of Cryptosporidium species isolated from cattle in Poland. Vet. Parasitol. 196, 301–306 (2013).
Google Scholar
-
Majewska, A. C., Werner, A. & Sulima, P. Występowanie kryptosporidiozy u bydła hodowlanego w jednym gospodarstwie rolnym – całoroczne badania. Ann. Parasitol. 47(Suppl 2), 31 (2001).
-
Bajer, A., Bednarska, M. & Siński, E. Molecular studies of Cryptosporidium spp. infections. Med. Weter. 61, 543–547 (2005).
-
Bornay-Llinares, F. J. et al. Identification of Cryptosporidium felis in a cow by morphologic and molecular methods. Appl. Environ. Microbiol. 65, 1455–1458 (1999).
Google Scholar
-
GUS, Working Group of National Agricultural Census 2010 (PSR 2010). Livestock and selected elements of animal production methods. Zakład Wydawnictw Statystycznych, Warszawa (2011).
-
Millar, C., Moore, J., Lowery, C., McCorry, K. & Dooley, J. Successful PCR amplification of genomic DNA from Cryptosporidium parvum oocysts extracted from a human faecal sample: A rapid and simple method suited for outbreak analysis. Int. J. Hyg. Environ. Health 204, 191–194 (2001).
Google Scholar
-
Xiao, L. et al. Phylogenetic analysis of Cryptosporidium parasites based on the small-subunit rRNA gene locus. Appl. Environ. Microbiol. 65, 1578–1583 (1999).
Google Scholar
-
Kaupke, A. & Rzeżutka, A. Population genetics of Cryptosporidium parvum subtypes in cattle in Poland: The geographical change of strain prevalence and circulation over time. BMC Vet. Res. 18, 263. https://doi.org/10.1186/s12917-022-03328-y (2022).
Google Scholar
-
Zintl, A. et al. Prevalence of Cryptosporidium species in intensively farmed pigs in Ireland. Parasitology 134, 1575–1582 (2007).
Google Scholar
-
Feng, Y. et al. Wide geographic distribution of Cryptosporidium bovis and the deer-like genotype in bovines. Vet. Parasitol. 144, 1–9 (2007).
Google Scholar
-
Kassouha, M., Soukkarieh, Ch. & Alkhaled, A. First genotyping of Cryptosporidium spp. in pre-weaned calves, broiler chickens and children in Syria by PCR-RFLP analysis. Vet. Parasitol. 225, 86–90 (2016).
Google Scholar
-
R Core Team R. A Language and Environment for Statistical Computing, R Foundation for Statistical Computing, Vienna, Austria 2021. https://www.R-project.org.
-
Devleesschauwer, B. et al. Tools for prevalence assessment studies, Package version 0.4.1. http://prevalence.cbra.be/ (2022).
-
Stevenson, M. et al. Tools for the analysis of epidemiological data, Package version 2.0.65. https://mvs.unimelb.edu.au/research/groups/veterinary-epidemiology-melbourne (2023).
-
Kváč, M., Hromadová, N., Květoňová, D., Rost, M. & Sak, B. Molecular characterization of Cryptosporidium spp. in pre-weaned dairy calves in the Czech Republic: Absence of C. ryanae and management-associated distribution of C. andersoni, C. bovis and C. parvum subtypes. Vet. Parasitol. 177, 378–382 (2011).
Google Scholar
-
Ondrácková, Z., Kvác, M., Sak, B., Kvetonová, D. & Rost, M. Prevalence and molecular characterization of Cryptosporidium spp. in dairy cattle in South Bohemia, the Czech Republic. Vet. Parasitol. 165, 141–144 (2009).
Google Scholar
-
Li, F. et al. Prevalence and molecular characterization of Cryptosporidium spp. and Giardia duodenalis in dairy cattle in Beijing, China. Vet. Parasitol. 219, 61–65 (2016).
Google Scholar
-
Němejc, K. et al. Occurrence of Cryptosporidium suis and Cryptosporidium scrofarum on commercial swine farms in the Czech Republic and its associations with age and husbandry practices. Parasitol. Res. 112, 1143–1154 (2013).
Google Scholar
-
Björkman, C. et al. Cryptosporidium infections in suckler herd beef calves. Parasitology 142, 1108–1114 (2015).
Google Scholar
-
Mirhashemi, M. E. et al. Molecular epidemiology of Cryptosporidium species in livestock in Ireland. Vet. Parasitol. 216, 18–22 (2016).
Google Scholar
-
Feng, Y. et al. Prevalence and genotypic identification of Cryptosporidium spp., Giardia duodenalis and Enterocytozoon bieneusi in pre-weaned dairy calves in Guangdong, China. Parasites Vectors 12, 41. https://doi.org/10.1186/s13071-019-3310-5 (2019).
Google Scholar
-
Fayer, R., Santín, M. & Dargatz, D. Species of Cryptosporidium detected in weaned cattle on cow–calf operations in the United States. Vet. Parasitol. 170, 187–192 (2010).
Google Scholar
-
Wang, Y. et al. Prevalence and molecular characterization of Cryptosporidium spp. and Giardia duodenalis in dairy cattle in Gansu, northwest China. Parasite 27, 62. https://doi.org/10.1051/parasite/2020058 (2020).
Google Scholar
-
Díaz, P. et al. Molecular characterisation and risk factor analysis of Cryptosporidium spp. in calves from Italy. Parasitol. Res. 117, 3081–3090 (2018).
Google Scholar
-
Lichtmannsperger, K. et al. Cryptosporidium parvum, Cryptosporidium ryanae, and Cryptosporidium bovis in samples from calves in Austria. Parasitol. Res. 119, 4291–4295 (2020).
Google Scholar
-
Holzhausen, I., Lendner, M., Göhring, F., Steinhöfel, I. & Daugschies, A. Distribution of Cryptosporidium parvum gp60 subtypes in calf herds of Saxony, Germany. Parasitol. Res. 118, 1549–1558 (2019).
Google Scholar
-
Díaz, P. et al. The age-related Cryptosporidium species distribution in asymptomatic cattle from North-Western Spain. Animals 11, 256. https://doi.org/10.3390/ani11020256 (2021).
Google Scholar
-
Imre, K. et al. Molecular characterisation of Cryptosporidium isolates from pre-weaned calves in Romania: Is there an actual risk of zoonotic infections?. Vet. Parasitol. 181, 321–324 (2011).
Google Scholar
-
Rieux, A., Paraud, C., Pors, I. & Chartier, C. Molecular characterization of Cryptosporidium isolates from pre-weaned calves in western France in relation to age. Vet. Parasitol. 197, 7–12 (2013).
Google Scholar
-
Smith, R. P., Clifton-Hadley, F. A., Cheney, T. & Giles, M. Prevalence and molecular typing of Cryptosporidium in dairy cattle in England and Wales and examination of potential on-farm transmission routes. Vet. Parasitol. 204, 111–119 (2014).
Google Scholar
-
Toledo, R. D. et al. Cryptosporidium spp. and Giardia spp. in feces and water and the associated exposure factors on dairy farms. PLoS ONE. 12, e0175311. https://doi.org/10.1371/journal.pone.0175311 (2017).
Google Scholar
-
Santín, M. et al. Prevalence and age-related variation of Cryptosporidium species and genotypes in dairy calves. Vet. Parasitol. 122, 103–117 (2004).
Google Scholar
-
Manyazewal, A. et al. Prevalence, risk factors and molecular characterization of Cryptosporidium infection in cattle in Addis Ababa and its environs, Ethiopia. Vet. Parasitol. Reg. Stud. Reports 13, 79–84 (2018).
Google Scholar
-
Geurden, T. et al. Prevalence and genotyping of Cryptosporidium in three cattle husbandry systems in Zambia. Vet. Parasitol. 138, 217–222 (2006).
Google Scholar
-
Duranti, A. et al. Risk factors associated with Cryptosporidium parvum infection in cattle. Zoonoses Public Health 56, 176–182 (2009).
Google Scholar
-
Kváč, M., Kouba, M. & Vítovec, J. Age-related and housing-dependence of Cryptosporidium infection of calves from dairy and beef herds in South Bohemia, Czech Republic. Vet. Parasitol. 137, 202–209 (2006).
Google Scholar
-
Silverlås, C. & Blanco-Penedo, I. Cryptosporidium spp in calves and cows from organic and conventional dairy herds. Epidemiol. Infect. 141, 529–539 (2013).
Google Scholar
-
Hoque, S. et al. High occurrence of zoonotic subtypes of Cryptosporidium parvum in Cypriot dairy farms. Microorganisms 10, 531. https://doi.org/10.3390/microorganisms10030531 (2022).
Google Scholar
-
Zhang, X. X. et al. Prevalence and molecular characterization of Cryptosporidium spp. in dairy cattle, northwest China. Parasitol. Res. 114, 2781–2787 (2015).
Google Scholar
-
Lee, S. H. et al. Multilocus typing of Cryptosporidium spp. in young calves with diarrhoea in Korea. Vet. Parasitol. 229, 81–89 (2016).
Google Scholar
-
Åberg, M., Emanuelson, U., Troell, K. & Björkman, C. Infection dynamics of Cryptosporidium bovis and Cryptosporidium ryanae in a Swedish dairy herd. Vet. Parasitol. https://doi.org/10.1016/j.vpoa.2019.100010 (2019).
Google Scholar
-
Silverlås, C., Bosaeus-Reineck, H., Näslund, K. & Björkman, C. Is there a need for improved Cryptosporidium diagnostics in Swedish calves?. Int. J. Parasitol. 43, 155–161 (2013).
Google Scholar
-
Trotz-Williams, L. A. et al. Calf-level risk factors for neonatal diarrhea and shedding of Cryptosporidium parvum in Ontario dairy calves. Prev. Vet. Med. 82, 12–28 (2007).
Google Scholar
-
Azami, M., Moghaddam, D. D., Salehi, R. & Salehi, M. The identification of Cryptosporidium species (protozoa) in Ifsahan, Iran by PCR-RFLP analysis of the 18S rRNA gene. Mol. Biol. (Mosk) 41, 934–939 (2007).
Google Scholar
-
Fayer, R., Santín, M., Trout, J. M. & Greiner, E. Prevalence of species and genotypes of Cryptosporidium found in 1–2-year-old dairy cattle in the eastern United States. Vet. Parasitol. 135, 105–112 (2006).
Google Scholar
-
Geurden, T. et al. Molecular epidemiology with subtype analysis of Cryptosporidium in calves in Belgium. Parasitology 134, 1981–1987 (2007).
Google Scholar
-
Langkjaer, R. B., Vigre, H., Enemark, H. L. & Maddox-Hyttel, C. Molecular and phylogenetic characterization of Cryptosporidium and Giardia from pigs and cattle in Denmark. Parasitology 134, 339–350 (2007).
Google Scholar
-
Chen, F. & Huang, K. Prevalence and molecular characterization of Cryptosporidium spp. in dairy cattle from farms in China. J. Vet. Sci. 13, 15–22 (2012).
Google Scholar
-
Cardona, G. A. et al. Unexpected finding of feline-specific Giardia duodenalis assemblage F and Cryptosporidium felis in asymptomatic adult cattle in Northern Spain. Vet. Parasitol. 209, 258–263 (2015).
Google Scholar
-
Majewska, A. C., Jędrzejewski, S., Słodkowicz-Kowalska, A., Solarczyk, P. & Werner, A. Epidemia kryptosporydiozy na farmie bydła mlecznego. Ann. Parasitol. 50, 70 (2004).
-
Kaupke, A., Michalski, M. M. & Rzeżutka, A. Diversity of Cryptosporidium species occurring in sheep and goat breeds reared in Poland. Parasitol. Res. 116, 871–879 (2017).
Google Scholar
Acknowledgements
The authors are grateful to all the veterinary surgeons who helped with sample collection and to Dorota Fijoł for her technical assistance.
Author information
Authors and Affiliations
Contributions
A.K conceived the original idea, performed the molecular assays, compiled and analysed results as well as assisted in drafting of the manuscript. A.R conceived the original idea, compiled and analysed the results, drafted, reviewed and corrected the manuscript. A.K and A.R approved final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
Reprints and Permissions
About this article
Cite this article
Rzeżutka, A., Kaupke, A. Cryptosporidium infections in asymptomatic calves up to 4 months in Poland: a cross-sectional population study.
Sci Rep 13, 20997 (2023). https://doi.org/10.1038/s41598-023-47810-5
-
Received: 10 July 2023
-
Accepted: 18 November 2023
-
Published: 28 November 2023
-
DOI: https://doi.org/10.1038/s41598-023-47810-5
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.