Cardiovascular
Medtronic debuts new FDA-cleared LAA exclusion device
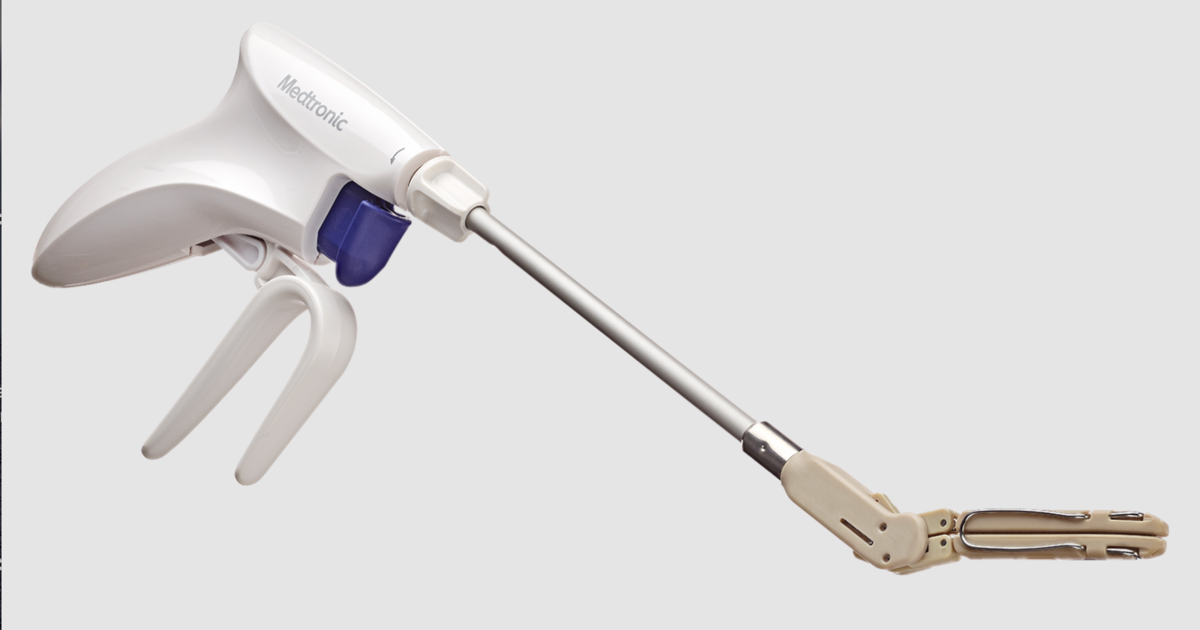
Medtronic has added a new device, the Penditure Left Atrial Appendage (LAA) Exclusion System, to its cardiac surgery portfolio. The device was designed for the exclusion of the LAA in patients undergoing concomitant heart surgeries, a step intended to help reduce their risk of stroke. It arrives pre-loaded on a single-use delivery system.
“The strategic addition of the Penditure Left Atrial Appendage Exclusion System demonstrates our commitment to investing in cardiac surgeons and their growing needs for managing patients with more complex cardiac disease,” Karim Bandali, PhD, president of Medtronic’s cardiac surgery business, said in a statement. “The Penditure device reinforces our commitment to innovation and provides an important, new, differentiated LAA management option for cardiac surgeons in the care of their patients.”
“This launch brings innovation to the space, offering a solution that is low profile and can provide more control and visibility than ever before,” added Gorav Ailawadi, MD, , director of the Frankel Cardiovascular Center at the University of Michigan in Ann Arbor and one of the first U.S. surgeons to use the device. “And while we hope we don’t need to use it often, the Penditure clip is recapturable and redeployable should we ever want to reposition an already deployed clip, which is a nice safety feature.”
