Infection
Pathogen species are the risk factors for postoperative infection of patients with transurethral resection of the prostate: a retrospective study
Abstract
This study aimed to analyze the infection risk factors for transurethral resection of the prostate (TURP) and establish predictive models to help make personalized treatment plans. Our study was designed one-center and retrospectively enrolled 1169 benign prostatic hyperplasia (BPH) patients. Risk factors were explored for postoperative infection. A TURP-postoperative infection (TURP-PI) model with infection prediction values was created. The improved-TURP-PI (I-TURP-PI) model, including extra new factors (pathogens species), was also built to see whether it could optimize the prediction abilities. At last, we developed a nomogram for better clinical application. Operation time, preoperative indwelling urinary catheter (PIUC), and positive preoperative urine culture were independent risk factors (all P < 0.05). Interestingly, pathogens species in pre-surgery urine (PEnterococcus faecium = 0.014, PPseudomonas aeruginosa = 0.086) were also independent risk factors. Patients with positive Enterococcus faecium (37.50%) were most likely to have postoperative infection. We built two models with AUCTURP-PI = 0.709 (95% CI 0.656–0.763) and AUCI-TURP-PI = 0.705 (95% CI 0.650–0.760). The nomogram could help improve the prediction ability. To our knowledge, our study is the first to use pathogen species in urine before surgery as risk factors for infection prediction after TURP. TURP-PI and I-TURP-PI models have essential roles in predicting patients’ postoperative infections and in better postoperative antibiotic decision-making.
Introduction
Benign prostatic hyperplasia (BPH) is a common condition that could affect the life quality of a man over 50. Roughly half of all men will suffer from BPH-related symptoms later in life1,2. Transurethral resection of the prostate (TURP) is a gold-standard surgery procedure for BPH patients 3. Although TURP is generally considered safe, effective, and well-tolerated, it is also associated with numerous complications, including infection, transurethral resection syndrome, bleeding, and prolonged hospitalization4,5. Studies showed that the infection rate after TURP ranged from 2.6 to 13.5% 6,7,8,9. A postoperative infection will prolong hospital stay, increase medical expenses, and affect patients’ quality of life. In addition, there will be a 0.7–4.4% chance of progressing to sepsis, which could endanger patients’ life 8,9,10. Studies showed that postoperative infection was related to factors such as positive preoperative urine culture, the preoperative indwelling urinary catheter (PIUC), duration of operation time, diabetes, age, use of prostate-related drugs, preoperative nutritional status, and antibiotics usage8,11,12. Elevated prostate-specific antigen (PSA) level may also be indicative of undetected infections in BHP patients 13.
As far as we know, there may not be comprehensive guidelines on postoperative infection prediction of TURP from the European Association of Urology (EAU) or the American Urological Association (AUA). Also, there is a lack of high-risk pathogen species and predictive models for postoperative infection after TURP in the literature. This study aimed to analyze the risk factors of infection susceptibility for BHP patients with TURP and establish warning scoring models to provide a basis for preventing and treating postoperative urinary infections.
Results
Clinical characteristics
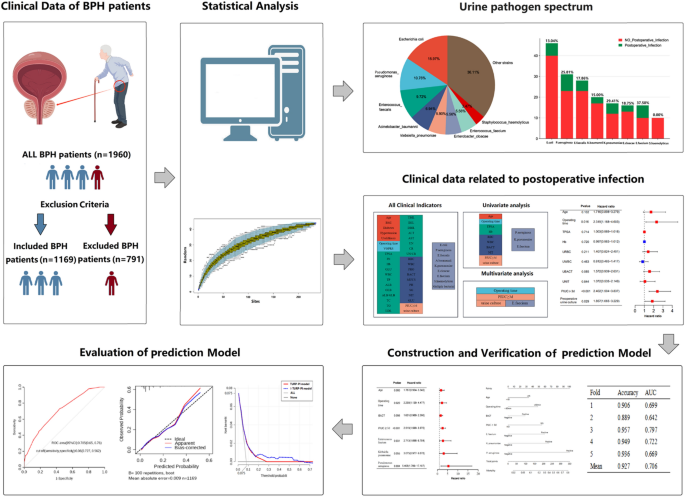
The workflow is shown in Fig. 1. This study’s median age was 70 (65, 75) years old, and the median BMI was 23.14 (21.22, 25.05) kg/m2. Among 1169 patients, 188 (16.08%) got diabetes, 466 (39.86%) had hypertension, 157 (13.43%) had upper urinary tract stones, and 319 (27.29%) retained urinary catheters for more than three days before surgery. The median operation duration time was 87 (62, 117) min. The median removed volume of the prostate was 20.8 (9.8, 41.6) ml. The details are shown in Table S1.
The wokflow of the study.
Risks for postoperative infection
Univariate analysis showed that postoperative infection was related to age, operation duration, preoperative total prostate-specific antigen (TPSA), preoperative hemoglobin (Hb), preoperative urine red blood cell microscopy (URBC), preoperative urine white blood cell microscopy (UWBC), preoperative urine bacteria (UBACT) were quantitative, preoperative urine nitrite qualitative, PIUC and positive preoperative urine culture (all P < 0.05, Tables 1, S2–6). Operation duration, indwelling catheter, and positive preoperative urine culture pre-surgery were independent risk factors for post-surgery infection for BHP patients (all P < 0.05, Table 1). The coefficient of each risk factor is shown in Fig. S1.
Urine pahtogen spectrum
235 (20.10%) patients had positive urine culture before the operation, 88 (7.53%) patients developed infections after TURP, and 44 (3.76%) had multiple infections. The species accumulation curve indicated that the sampling was sufficient for data analysis (Fig. 2A). The most common pathogens were Escherichia coli (15.97%), followed by Pseudomonas aeruginosa (10.76%) and Enterococcus faecalis (9.72%) (Fig. 2B, Table 2). Furthermore, Enterococcus faecium was an independent risk factor for postoperative infection by multivariate analysis (P = 0.014). The details are showed in Fig. 2C,D and Table 2.

Distribution of urine pathogen. (A) Species accumulation curve. (B) Proportion of different pathogens in preoperative urine culture. (C) Incidence of postoperative infection in different pathogen-positive groups. (D) Comparison of detection rates of different pathogens in non_postoperative and postoperative infection groups.
Predictive model construction and validation
Risk factors to build the TURP-postoperative infection (TURP-PI) and improved-TURP-PI (I-TURP-PI) models were shown in Table S7. Each factor’s coefficient is shown in Fig. S2. The nomogram exhibited the models’ application (Fig. 3A,D). The two models were stable and credible by fivefold cross-validation (Table S8). The area under the curve (AUC) of the two models were above 0.7 (AUCTURP-PI = 0.709 (95% CI 0.656–0.763), AUCI-TURP-PI = 0.705 (95% CI 0.650–0.760) (Fig. 3B,E). Then, calibration curves showed they were reliable by the Hosmer–Lemeshow test (PTURP-PI = 0.983, PI-TURP-PI = 0.988). Also, the decision curves showed the superiority of the I-TURP-PI model based on the net benefit and threshold probabilities (1.2–62.7%), compared to the TURP-PI model (1.8–26.3%) (Fig. 3C,F), indicating I-TURP-PI models could benefit more extensive patients. (Fig. 3G).

Nomogram for two predictive models. (A) TURP-PI model; (B) ROC of TURP-PI model; (C) The calibration curve of TURP-PI model; (D) I-TURP-PI model; (E) The ROC of I-TURP-PI model; (F) The calibration curve of I-TURP-PI model; (G) The clinical impact curves of two models.
Discussion
TURP has been the standard golden treatment for BPH patients for over a century since its invention 14. Our previous study showed that, for patients with upper urinary tract stones, bacterial species of preoperative urine culture impact the post-surgery infection after percutaneous nephrolithotomy (PCNL) 15. However, as far as we know, its role in infection prediction of patients with TURP hasn’t been studied in the literature. We found that specified types of infected pathogens in urine before surgery can influence the postoperative infection rate of TURP.
In our study, we found patients with positive preoperative urine culture were more than 2.5 times likelihood to develop postoperative infections, compared with patients with negative one (14.47% VS 5.78%). Patients infected with Enterococcus faecium were most susceptible to infection after TRUP, followed by Klebsiella pneumoniae and Pseudomonas aeruginosa. These three pathogens were also reported to be responsible for secondary bacteremia in children with symptomatic nosocomial urinary tract infections 16. By reading the extensive literature, we found that all three bacteria have their own characteristics. Enterococcus faecium is resistant to many antimicrobial agents and could cause serious incidence rates and mortality 17,18,19. Klebsiella pneumoniae has strong hidden abilities and invasiveness, leading to immune escape and resistance to urine erosion 20,21. It could cause urinary tract infections, pneumonia, liver abscess, surgical site infections, and bloodstream infections 22. Pseudomonas aeruginosa is an antibiotic-resistant pathogen of great concern to the World Health Organization (WHO)23. It has many resistance mechanisms, such as antibiotic-modifying enzymes, obtaining plasmids encoding drug-resistance genes, limited penetration of antibiotics, and the possibility of generating energy-dependent pumps 24. Elderly patients infected by Pseudomonas aeruginosa could even relapse after antibiotic treatment 25. Understanding the characteristics of these bacteria could help clinicians pay more attention to specific bacteria in preoperative urine culture to better infection prevention and rational use of antibiotics.
Our research shows that some species are commonly detected in urinary tract infections, such as Escherichia coli and Enterococcus faecalis. But they don’t have the solid pathogenicity to induce infection after surgery. While for some specific species, even with strong antibiotics pre-surgery, patients are still prone to get infections9. There are two main reasons for this confusion phenomenon. For bacteria, some certain kinds are easily cultured and resistant to drugs, leading a strong pathogenicity 19,26. They “escape” from surveillance of antibiotics in many ways, such as cell stress responses, biofilm formation, and genetic material changes22,24,27. For the host, BPH usually happens in older men with poor self-care abilities and the weakened immune system. Preoperative positive urine culture with certain bacteria in BPH patients should be taken seriously to reduce the infection after surgery.
Besides positive preoperative urine culture, operation time and PIUC were also independent risk factors for infection after TURP, which is in accordance with previous studies 28,29,30. The application of PICU could not only induce biofilm formation but also impair the defense mechanisms of the urinary tract, making patients susceptible to infection 31. A previous study also confirmed once organisms gained access to the catheterized urinary tract, low-level bacteriuria could rapidly progress to high-level in 24–48 h 32.
We established two models with many influence clinical parameters to optimize their prediction power. Although the two models had similar prediction abilities and model fitting degrees, the I-TURP-PI model could benefit more BPH patients than the TURP-PI model. Most importantly, when constructing the I-TURP-PI model, we excluded “urine culture” from multivariate analysis. Instead, pathogens species in urine culture were then included in the model construction, indicating specific pathogens in urine were the key factors affecting postoperative infection.
The highlight of our research is that, for the first time, we found the specific types of pathogens in preoperative urine could increase the infection probability after TURP. We then used the pathogen species as the evaluation index for model-building to predict infection after TURP. In clinical practice, it often takes 2–3 days to obtain a post-operative urine culture result, leading to untimely administration of appropriate antibiotics. Thus, advance knowledge of common urinary tract bacteria and their corresponding characteristics of infection could be of great help in clinical diagnosis and treatment.
However, there were some limitations. Firstly, our study was a retrospective and one-center design. The data might be biased. Prospective clinical trials with more extensive patients were needed. Secondly, the antibiotic resistance of pathogens varies in different regions, which may lead to some deviations. However, we can map the species’ spectrum in the various areas for proper antibiotic treatment. Thirdly, data such as Free-PSA and types of preoperative antibiotics, which may influence the incidence of postoperative infections, were not included in our model because they were unavailable.
Conclusion
The positive preoperative urine culture of Enterococcus faecium, Klebsiella pneumoniae, and Pseudomonas aeruginosa, could affect the postoperative infection of BPH patients with TURP. Our models might help predict the occurrence of postoperative infection and guide the use of antibiotics in clinical settings. Clinicians could focus more on pathogens species in preoperative urine cultures when making clinical decisions for patients undergoing TURP.
Materials and methods
Study population and data collection
Our study was a single-center and case–control design, approved by the Institutional Review Board of the Fujian Provincial Hospital Ethics Committee, in compliance with the Declaration of Helsinki (K2023-01-011). From January 2015 to June 2022, 1960 BPH patients who underwent TURP in the urology department of Fujian provincial hospital were enrolled. All BPH patients received prophylactic antibiotics during the perioperative period. We included patients whose urine cultures were initially positive but turned negative after antibiotic treatment. And diabetes patients whose fasting plasma glucose was controlled below eight mmol/L. Patients with bladder stones or tumors, blood system diseases, immune system diseases, other system infections (< 1 mouth), patients undergoing other operations during the same period, and patients without complete clinical information were excluded. Finally, 1169 patients were included in our study. The patient selection is shown in Fig. S3.
The following data were collected: (1) preoperative information: age, BMI, complications, laboratory tests. (2) operative information: operating time and volume of surgically removed prostate. (3) postoperative information such as maximum temperature and blood culture.
Criteria of infection
Postoperative infection was defined as positive blood culture or body temperature above 38.5℃. Other systemic infections or unknown fevers were excluded.
Risks for postoperative infection
Thirty-eight clinical parameters were included for univariate analysis, such as indwelling catheter before surgery, the preoperative urine culture, and operating time. Then multivariate analysis was performed to identify independent risk factors that could affect post-surgery infection.
Urine pathogen spectrum
We performed univariate analysis to analyze the relationship between pathogen species and infection. Species accumulation curves were plotted.
Identification and validation of the predictive models
We first used the risk factors which significantly affect postoperative infection by univariate analysis to construct a predictive model (TURP-PI model). Then “pathogen species” was added to the TURP-PI model to create an improved-TURP-PI model (I-TURP-PI) to see whether pathogen species could enhance the prediction ability. Furthermore, the two models were compared and assessed by receiver operator characteristic curve (ROC) curves, the Hosmer–Lemeshow test, and decision curve analysis. They were also validated by five-fold cross-validation.
Risk score
We calculated each patient’s risk score based on the formula below: Risk score TURP-PI = − 4.451 + (0.567) × (age) + (0.845) × (operation time) + (0.477) × (UBACT) + (0.905) × (PIUC ≥ 3d) + (0.626) × (Preoperative urine culture), Risk score I-TURP-PI = − 0.570 + (0.569) × (age) + (0.814) × (operation time) + (0.471) × (UBACT) + (0.924) × (PIUC ≥ 3d) + (0.998) × (Enterococcus faecium) + (1.123) × (Klebsiella pneumoniae) + (1.688) × (Pseudomonas aeruginosa).
Statistical analysis
SPSS (16.0 version) was used to analyze data. The t-test, a rank-sum test, chi-square test, and logistic regression were performed to analyze data. P < 0.05 was statistically significant. Visualization was performed through R software (version 3.6.1).
Ethical approval
Our study was approved by the Institutional Review Board of the Fujian Provincial Hospital Ethics Committee, in compliance with the Declaration of Helsinki (K2023-01-011).
Consent to participate
This study was retrospective and approved by the Ethics Committee of Fujian Provincial Hospital. The exemption from the Informed Consent Form was approved by the Ethics Committee of Fujian Provincial Hospital.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request, provided that no copy rights are violated.
Abbreviations
- BPH:
-
Benign prostatic hyperplasia
- TURP:
-
Transurethral resection of the prostate
- PIUC:
-
Preoperative indwelling urinary catheter
- PSA:
-
Prostate-specific antigen
- IQR:
-
Interquartile range
- TPSA:
-
Prostate-specific antigen
- PLT:
-
Platelet
- Hb:
-
Hemoglobin
- GLU:
-
Glucose
- WBC:
-
White blood cell
- TP:
-
Total protein
- ALB:
-
Albumin
- GLB:
-
Globulin
- ALB/GLB:
-
Albumin/globulin
- TC:
-
Total cholesterol
- TG:
-
Triglyceride
- LDL:
-
Low-density lipoprotein
- TBIL:
-
Total bilirubin
- IBIL:
-
Indirect bilirubin
- DBIL:
-
Direct bilirubin
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate transaminase
- UN:
-
Urea nitrogen
- CR:
-
Creatinine
- UN/CR:
-
Urea nitrogen/creatinine
- URBC:
-
Urine red blood cell
- UWBC:
-
Urine white blood cell
- UBACT:
-
Urine bacteria
- UNIT:
-
Urine nitrite
- PCNL:
-
Percutaneous nephrolithotomy
- ROC:
-
Receiver operator characteristic curve
- WHO:
-
World Health Organization
References
-
Dornbier, R., Pahouja, G., Branch, J. & McVary, K. T. The New American Urological Association benign prostatic hyperplasia clinical guidelines: 2019 update. Curr. Urol. Rep. 21, 32 (2020).
Google Scholar
-
Zhu, C. et al. Epidemiological trends of urinary tract infections, urolithiasis and benign prostatic hyperplasia in 203 countries and territories from 1990 to 2019. Mil. Med. Res. 8, 64 (2021).
Google Scholar
-
Lerner, L. B. et al. Management of lower urinary tract symptoms attributed to benign prostatic hyperplasia: AUA GUIDELINE PART II-surgical evaluation and treatment. J. Urol. 206, 818–826 (2021).
Google Scholar
-
Rassweiler, J., Teber, D., Kuntz, R. & Hofmann, R. Complications of transurethral resection of the prostate (TURP)—Incidence, management, and prevention. Eur. Urol. 50, 969–979 (2006) (discussion 980).
Google Scholar
-
Sun, F., Sun, X., Shi, Q. & Zhai, Y. Transurethral procedures in the treatment of benign prostatic hyperplasia: A systematic review and meta-analysis of effectiveness and complications. Medicine 97, e13360 (2018).
Google Scholar
-
Ahyai, S. A. et al. Meta-analysis of functional outcomes and complications following transurethral procedures for lower urinary tract symptoms resulting from benign prostatic enlargement. Eur. Urol. 58, 384–397 (2010).
Google Scholar
-
Qiang, W., Jianchen, W., MacDonald, R., Monga, M. & Wilt, T. J. Antibiotic prophylaxis for transurethral prostatic resection in men with preoperative urine containing less than 100,000 bacteria per ml: A systematic review. J. Urol. 173, 1175–1181 (2005).
Google Scholar
-
Riedinger, C. B. et al. The impact of surgical duration on complications after transurethral resection of the prostate: An analysis of NSQIP data. Prostate Cancer Prostatic Dis. 22, 303–308 (2019).
Google Scholar
-
Schneidewind, L. et al. Mulitcenter study on antibiotic prophylaxis, infectious complications and risk assessment in TUR-P. Cent. Eur. J. Urol. 70, 112–117 (2017).
Google Scholar
-
Berry, A. & Barratt, A. Prophylactic antibiotic use in transurethral prostatic resection: A meta-analysis. J. Urol. 167, 571–577 (2002).
Google Scholar
-
Kikuchi, M. et al. Postoperative infectious complications in patients undergoing holmium laser enucleation of the prostate: Risk factors and microbiological analysis. Int. J. Urol. 23, 791–796 (2016).
Google Scholar
-
Li, Y.-H. et al. Clinical analysis of urinary tract infection in patients undergoing transurethral resection of the prostate. Eur. Rev. Med. Pharmacol. Sci. 21, 4487–4492 (2017).
Google Scholar
-
Ivanov, S. N., Kogan, M. I., Naboka, Y. L. & Medvedev, V. L. Infectious factor in transuretral surgery of benign prostate hyperplasia: a systematic review and meta-analysis. Urologiia 4, 141–149 (2023).
-
Jardin, A. Brief history of BPH management. Progres en urologie: journal de l’Association francaise d’urologie et de la Societe francaise d’urologie 28, 799–802 (2018).
Google Scholar
-
Yang, Z. et al. The effect of preoperative urine culture and bacterial species on infection after percutaneous nephrolithotomy for patients with upper urinary tract stones. Sci. Rep. 12, 4833 (2022).
Google Scholar
-
Devrim, F. et al. Bacteremia due to healthcare-associated urinary tract infections in children. Archives de pediatrie : organe officiel de la Societe francaise de pediatrie 28, 147–149 (2021).
Google Scholar
-
García-Solache, M. & Rice, L. B. the enterococcus: A model of adaptability to its environment. Clin. Microbiol. Rev. 32, e00058-18 (2019).
Google Scholar
-
Jabbari Shiadeh, S. M., Pormohammad, A., Hashemi, A. & Lak, P. Global prevalence of antibiotic resistance in blood-isolated Enterococcus faecalis and Enterococcus faecium: A systematic review and meta-analysis. Infect. Drug Resist. 12, 2713–2725 (2019).
Google Scholar
-
Wang, Q.-Y., Li, R.-H. & Shang, X.-H. Urinary tract infection caused by Enterococcus isolates: Aetiology and antimicrobial resistance patterns. J. Chemother. 27, 117–119 (2015).
Google Scholar
-
Caneiras, C., Lito, L., Melo-Cristino, J. & Duarte, A. Community- and hospital-acquired Klebsiella pneumoniae urinary tract infections in portugal: Virulence and antibiotic resistance. Microorganisms 7, 138 (2019).
Google Scholar
-
Lin, W. H. et al. Clinical and microbiological characteristics of Klebsiella pneumoniae from community-acquired recurrent urinary tract infections. Eur. J. Clin. Microbiol. Infect. Dis. 33, 1533–1539 (2014).
Google Scholar
-
Wang, G., Zhao, G., Chao, X., Xie, L. & Wang, H. The characteristic of virulence, biofilm and antibiotic resistance of Klebsiella pneumoniae. Int. J. Environ. Res. Public Health 17, 6278 (2020).
Google Scholar
-
Tacconelli, E. et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 18, 318–327 (2018).
Google Scholar
-
Paz-Zarza, V. M. et al. Pseudomonas aeruginosa: Pathogenicity and antimicrobial resistance in urinary tract infection. Revista chilena de infectologia: organo oficial de la Sociedad Chilena de Infectologia 36, 180–189 (2019).
Google Scholar
-
Álvarez Artero, E. et al. Urinary infection in the elderly. Revista clinica espanola 219, 189–193 (2019).
Google Scholar
-
Kotov, S. V. et al. The problem of antibiotic resistance in patients with urinary tract infection. Urologiia 5–12 (2021).
-
Darnell, R. L. et al. Antimicrobial tolerance and its role in the development of resistance: Lessons from enterococci. Adv. Microb. Physiol. 81, 25–65 (2022).
Google Scholar
-
Huang, X. et al. Bacteriuria after bipolar transurethral resection of the prostate: Risk factors and correlation with leukocyturia. Urology 77, 1183–1187 (2011).
Google Scholar
-
Hwang, E. C. et al. A prospective Korean multicenter study for infectious complications in patients undergoing prostate surgery: Risk factors and efficacy of antibiotic prophylaxis. J. Korean Med. Sci. 29, 1271–1277 (2014).
Google Scholar
-
Osman, T. et al. Evaluation of the risk factors associated with the development of post-transurethral resection of the prostate persistent bacteriuria. Arab J. Urol. 15, 260–266 (2017).
Google Scholar
-
Trautner, B. W. & Darouiche, R. O. Role of biofilm in catheter-associated urinary tract infection. Am. J. Infect. Control 32, 177–183 (2004).
Google Scholar
-
Stark, R. P. & Maki, D. G. Bacteriuria in the catheterized patient. What quantitative level of bacteriuria is relevant?. N. Engl. J. Med. 311, 560–564 (1984).
Google Scholar
Funding
This work was supported by the Natural Science Foundation of Fujian Province (Grant number 2021J01398) and The proj of 2022 Fujian Provincial Department of Finance (Fujian Finance NO.2022-840).
Author information
Authors and Affiliations
Contributions
J.L. and Z.Y.: conceptualization, data curation, formal analysis, methodology, writing original draft, revision, and editing. L.Y.: formal analysis, methodology, revision, and editing. Y.H. and W.C.: data curation, revision, and editing. H.P.: revision and editing of the final manuscript. H.F. and J.W.: data curation, writing the original draft, revision and editing of the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary Figures.
Supplementary Figure S3.
Supplementary Tables.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
Reprints and Permissions
About this article
Cite this article
Lin, J., Yang, Z., Ye, L. et al. Pathogen species are the risk factors for postoperative infection of patients with transurethral resection of the prostate: a retrospective study.
Sci Rep 13, 20943 (2023). https://doi.org/10.1038/s41598-023-47773-7
-
Received: 22 August 2023
-
Accepted: 18 November 2023
-
Published: 28 November 2023
-
DOI: https://doi.org/10.1038/s41598-023-47773-7
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.