Infection
Researchers develop material that reduces bacterial infection and speeds up bone healing
Researchers at RCSI University of Medicine and Health Sciences and Advanced Materials and Bioengineering Research Centre (AMBER) have developed a new surgical implant that has the potential to transform the treatment of complex bone infections. When implanted on an injured or infected bone, the material can not only speed up bone healing it also reduces the risk of infections without the need for traditional antibiotics.
The newly published paper in the journal Advanced Materials tackles the complex clinical problem of bone infection, or osteomyelitis, which affects one in around 5,000 people within the US only each year.
When a bone is infected, the priority is to heal it quickly. Standard clinical treatment, including several weeks with antibiotics and often removing the infected portion of bone tissue, can be slow. Already, around half of bone infections are caused by MRSA, which is resistant to antibiotics, and prolonged antibiotic treatment pushes up the risk of infections becoming tolerant to the treatments we have at our disposal, making infections harder to control.
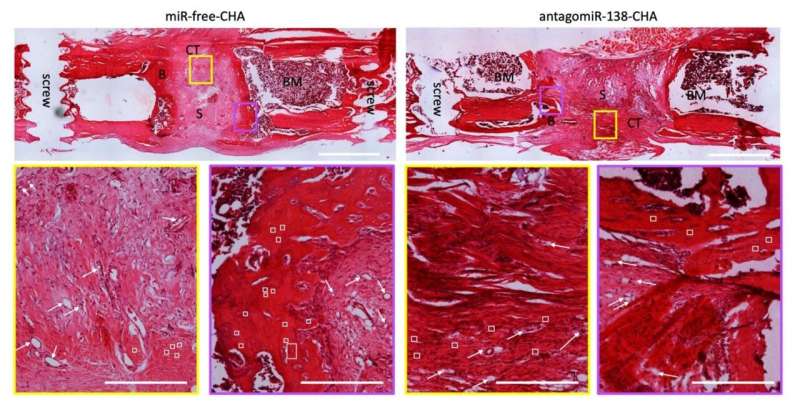
To help patients heal well, researchers at RCSI created a material from a substance that is similar to our bones. The scaffold-like structure of this material means that when it is implanted into injured or diseased bone, it encourages the bone to regrow.
In this case, the RCSI researchers infused the scaffold with tiny nanoparticles of copper, which are known to kill the bacterium that causes most bone infections. Furthermore, they also incorporated a specific genetic molecule, an inhibitor of microRNA-138, into the scaffold to stimulate the formation of new bone at the site where the material is implanted.
In the study, the researchers describe how preclinical lab tests showed the implanted scaffolds with the copper nanoparticles and microRNA could stimulate bone regrowth in a fortnight, and that the scaffold stopped 80% of potentially harmful bacteria from attaching to the site.
They also saw that the implants stimulated a good blood supply to cells on the scaffold, which is crucial for the health and viability of the newly formed bone.
“Overall, we combined the power of antimicrobial implants and gene therapies, leading to a holistic system which repairs bone and prevents infection,” says the first author of the study, Dr. Joanna Sadowska, a Marie Skłodowska-Curie Postdoctoral Fellow at the RCSI Tissue Engineering Research Group (TERG).
“This makes a significant step forward in treating complex bone injuries, and the timescale we saw in our preclinical studies suggests our approach could revolutionize treatment times for patients in the future.”
Professor of Bioengineering and Regenerative Medicine at RCSI, Prof. Fergal O’Brien, Principal Investigator on the paper and Head of TERG, sees many potential benefits to the implant.
“This implant can deliver the antimicrobial treatment directly to the infected bone, so it can be a local and targeted approach, as opposed to exposing more of the body to long-term antibiotics,” he says.
“Add to this that our implant incorporates copper particles that can stop bacteria from establishing an infection at the site, and at the same time they stimulate blood vessel formation in bone. The nature of the implant also means that the body can naturally break down the material when the bone heals, so there is no need to remove it surgically.”
Professor O’Brien, who is Deputy Director of AMBER, the SFI Centre for Advanced Materials and BioEngineering Research, sees the implant as an important future step mode of targeted delivery for more precise and effective treatments of injured and diseased bone.
“This is a first-of-its-kind implant that integrates different solutions to encourage bone regrowth and address infections, and the new study is an important step to bringing it toward patients for faster and more effective treatments,” he said.
More information:
Joanna M. Sadowska et al, A multifunctional scaffold for bone infection treatment by delivery of microRNA therapeutics combined with antimicrobial nanoparticles, Advanced Materials (2023). DOI: 10.1002/adma.202307639
Royal College of Surgeons in Ireland
Citation:
Researchers develop material that reduces bacterial infection and speeds up bone healing (2023, November 28)
retrieved 28 November 2023
from https://medicalxpress.com/news/2023-11-material-bacterial-infection-bone.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.