Cancer and neoplasms
Sustained Clinical Response to 4th-Line Therapy with Selpercatinib in
Introduction
The 2021 WHO classification categorizes neuroendocrine neoplasms (NENs) of the lung as a single tumor group, which includes low- and intermediate-grade typical carcinoid (TC) and atypical carcinoid (AC) and high-grade NE carcinomas (NECs), including large cell neuroendocrine carcinoma (LCNEC) and small cell lung cancer (SCLC).1 Combined small cell lung cancer (C-SCLC) is a subtype of SCLC that comprises three types according to the different components combined with SCLC, including adenocarcinoma, LCNEC, and squamous cell carcinoma. Indeed, approximately 5–28% of surgically resected SCLCs are diagnosed as C-SCLCs.2,3 However, limited specimens, such as those obtained for cytology or from a small biopsy, tend to underestimate C-SCLC incidence. The current guidelines for SCLC management do not describe a standard treatment for C-SCLC in detail. Routinely, C-SCLC management consists of multimodality treatment (surgery, radiotherapy, chemotherapy, and immunotherapy) and is often based on SCLC guidelines.4
To date, genomic changes in C-SCLC remain unclear, and RET fusions in C-SCLC have not been reported. The RET proto-oncogene is located on the long arm of chromosome 10 with a length of 60,000 bp and 21 exons.5 The RET gene promotes carcinogenesis primarily by gene fusion, point mutation, and amplification, and is associated with multiple cancers.6 RET fusions have been detected in most papillary thyroid carcinomas and in 1–2% of NSCLCs.7,8 RET fusions involve different fusion partners, with the most common being KIF5B-RET (62–68.2%) and CCDC6-5B (16.8–21%).9–12 Currently, the tyrosine kinase inhibitors (TKIs) selpercatinib and pralsetinib have been approved by the FDA for locally advanced or metastatic solid tumors with RET fusions that have progressed on or following prior systemic treatment.13,14 Furthermore, several strategies and novel targeted drugs are currently under investigation.15
Here, we report for the first time a case of extensive-stage C-SCLC, in which the tumor harbored the KIF5B-RET fusion before first-line therapy and was persistently sensitive to selpercatinib after failed cytotoxic chemotherapy, immunochemotherapy and anlotinib hydrochloride administration. This case highlights the importance of comprehensive molecular testing in C-SCLC for selecting the optimal treatment. Although RET fusion is rare in patients with C-SCLC, its identification and treatment using selective RET inhibitors may contribute to clinical benefits.
Case Presentation
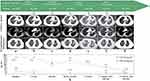
A 57-year-old never-smoking Asian woman with no significant medical history presented with cough and dyspnea for 2 months. Computed tomography (CT) revealed a mass in her left upper lung (primary lesion, 4.1 cm) with obvious bilateral intrapulmonary and mediastinal lymph node metastases (Figure 1a), without bone, liver, kidney, and brain metastases.
|
Figure 1 Imaging evaluation and detection of serum tumor biomarkers during treatment. (a–g) Sequence of anticancer treatments and the corresponding imaging evaluations. Red arrows indicate the primary tumor, while red asterisks indicate newly developed intrapulmonary metastatic lesions. A remarkable shrinkage of the primary tumor and intrapulmonary metastases was observed after 5 and 14 months of treatment with selpercatinib. (h) Serum CEA and NSE were detected to monitor clinical response during the treatment. Abbreviations: Jan., January; Mar., March; Jul., July; Dec., December; Aug., August; VP-16, etoposide; DDP, cisplatin; T, atezolizumab; Nab-p, nab-paclitaxel; CBP, carboplatin; PCI, prophylactic cranial irradiation; CEA, carcinoembryonic antigen; NSE, neuron-specific enolase. |
Subsequently, a transbronchial biopsy of the tumor mass in the left upper lobe was carried out, and histological analysis revealed C‐SCLC (Figure 2a) comprising SCLC (Figure 2b) and LCNEC (Figure 2c), without gland formation or keratinization. Immunohistochemistry (IHC) showed positive signals for synaptophysin (Syn, Figure 2d–f), pan-cytokeratin (PCK, Figure 2g–i), and Ki67 (MIB-1, Figure 2j–l), with a high proliferative index of 50% (average for SCLC and LCNEC). Other biomarkers, including napsin A, lymphocyte common antigen (LCA), and desmin were not detected. Finally, the patient was diagnosed with advanced stage (cT2bN2M1a, IVA) lung neuroendocrine carcinoma (combined SCLC and LCNEC) in January 2021.
|
Figure 2 Histological analysis of the transbronchial lung biopsy revealed two different tumor components. (a) Hematoxylin and eosin (H&E) staining showing C-SCLC (combined SCLC and LCNEC, 100×). (b) SCLC is characterized by the presence of diffuse sheets of small round-to-fusiform cells, with scant cytoplasm, and inconspicuous or no nucleoli with finely granular nuclear chromatin. Meanwhile, nuclear chromatin smearing, also termed crushed artifact, is common in SCLC (400×). (c) LCNEC is characterized by large cells, frequent presence of nucleoli and abundant cytoplasm (400×). (d–f) Both the SCLC and LCNEC components were positive for synaptophysin (Syn, 100×, 400×). (g) Different modes of PCK expression in C-SCLC (100×). (h) The so-called dot-like staining pattern of PCK in SCLC (400×). (i) The LCNEC component was diffusely positive for PCK in the cytoplasm (400×). (j–l) Different Ki67 levels were found in both tumor components (100×, 400×). |
The patient showed progressive disease (PD) after 3 cycles of first-line chemotherapy (cisplatin, etoposide) combined with immunotherapy (atezolizumab), manifested as enlargement of the primary lesion (5.0 cm, Figure 1b). Considering the presence of LCNEC, paclitaxel-albumin combined with carboplatin was applied for second-line chemotherapy while maintaining atezolizumab. Fortunately, the patient achieved a partial response (PR) after four cycles (primary lesion, 2.6 cm; Figure 1c) and received prophylactic cranial irradiation (PCI) subsequently.
Then, the patient developed PD during regular follow-up with new intrapulmonary metastases (Figure 1d). Considering the Eastern Cooperative Oncology Group (ECOG) performance status (PS) of the case was 2 and the patient declined further tissue biopsy and chemotherapy, anlotinib hydrochloride, a novel multitarget tyrosine kinase inhibitor, was administered as third-line therapy. However, the lesions grew rapidly and additional metastases occurred (Figure 1e). To examine genetic changes in the tumors, next-generation sequencing (NGS), evaluating 1021 cancer-related genes (Geneplus),16 was performed on treatment-naive tumor and blood specimens after third-line treatment, respectively. NGS data showed that the treatment-naïve tumor from the patient had the KIF5B-RET fusion (41.4%) and RB1 deletion, with blood cell-free DNA (cfDNA) still containing the KIF5B-RET fusion (0.1%). Soon afterwards, the patient was administered 160 mg selpercatinib twice daily and achieved a PR in almost all lesions, including the primary tumor (3.1 cm) and metastatic lesions (Figure 1f).
At the time of manuscript preparation, the patient was still taking selpercatinib with a progression-free survival (PFS) surpassing 14 months and ongoing response (primary lesion, 2.8 cm; Figure 1g). No obvious drug-related adverse reactions have been observed thus far. Meanwhile, the monitoring of serum carcinoembryonic antigen (CEA) and neuron-specific enolase (NSE) during the treatment course revealed these biomarkers may be related to therapeutic effects (Figure 1h). Written informed consent was obtained from the patient for the publication of this case.
Discussion
This report presents a patient with stage IV C-SCLC harboring the KIF5B-RET fusion whose disease responded to selpercatinib for both primary tumor and intrapulmonary metastases in 4th-line treatment, highlighting the importance of comprehensive molecular testing in C-SCLC for selecting the optimal treatment. To the best of our knowledge, this is the first report demonstrating the presence of the KIF5B-RET fusion and examining the effect of RET inhibitor therapy in stage IV C-SCLC.
To date, the genomic profiling of C-SCLC has been rarely examined, and several studies have reported the molecular characterization of C-SCLC by NGS.17–19 In the latter reports, the most altered genes were tumor protein P53 (TP53) and retinoblastoma 1 (RB1), followed by KRAS and NOTCH family genes. In addition, targetable genomic mutations include EGFR and ALK alterations. There are also detected in C-SCLCs, which usually contain the adenocarcinoma component.20 However, RET fusions have not been reported in C-SCLC. Herein, we present a patient with stage IV C-SCLC harboring the KIF5B-RET fusion.
Overall, RET mutations in SCLC are rare. Mulligan et al and Lu et al identified no mutations with statistical significance in the RET gene in 32 SCLC patients and 54 SCLC cell lines, respectively.21,22 Similarly, RET fusions have not been reported in C-SCLC.17–19 However, RET fusions could be found in pure LCNEC occasionally. For instance, in a study examining 52 LCNEC patients by reverse transcription-polymerase chain reaction (RT-PCR), one patient had a RET fusion.23 In addition, Aakriti et al reported a case of stage IV LCNEC harboring the KIF5B-RET fusion who responded to selpercatinib.24 In the current case, considering CEA level fluctuation throughout the course of treatment and the limitations of needle biopsy in this non-smoking Asian female patient, the adenocarcinoma component25 or driver genes could not be ruled out. Therefore, NGS was performed, and the KIF5B-RET fusion was confirmed. Therefore, clinicians should pay close attention to CEA levels in patients with neuroendocrine cancer throughout the disease course and be aware of the possibility of driver genes in C-SCLC.
Selpercatinib is a first-in-class, highly selective and potent RET kinase inhibitor with central nervous system (CNS) activity.26 To date, selpercatinib has been approved in multiple countries for advanced or metastatic RET-altered lung cancers. The Phase I/II LIBRETTO-001 trial is a single-arm open-label study of selpercatinib in patients with RET-altered NSCLC. Updated efficacy data from this study in platinum chemotherapy pretreated patients (n = 247) indicated an objective response rate (ORR) of 61% (95% CI: 55 to 67), with 7% of cases achieving a complete response (CR) and a median duration of response (DoR) and PFS of 28.6 and 24.9 months, respectively.27 Meanwhile, selpercatinib also showed low-grade toxicity in LIBRETTO-001 and the SIREN study, a real-world retrospective study,28 providing a basis for the use of selpercatinib in patients with poor PS (≥2). Similarly, the C-SCLC patient with the RET-fusion (PS = 2) in this report achieved durable efficacy with low-grade toxicity in 4th-line treatment. Notably, no intracranial metastasis was found at regular cranial MRI/CT follow-ups (data not shown). This may be due to the timely PCI and selpercatinib (CNS activity).26 In this real-world study, the selective RET-inhibitor selpercatinib demonstrated durable systemic effects in RET fusion-positive C-SCLC and was well tolerated.
Regarding patient prognosis in C-SCLC, conflicting results have been reported by various studies. While a worse OS was reported in C-SCLC cases compared with individuals with pure SCLC,17,18 others reported that C-SCLC patients have better OS.29 Yang et al found marginally nonsignificant differences in OS (median: C-SCLC, 10.00 vs pure SCLC, 9.00 months; P = 0.17) based on the SEER database.30 Although the current C-SCLC case had progressed to stage IV at the initial diagnosis, her survival time far exceeded the median overall survival times of previously reported C-SCLC patients with active and reasonable treatment strategies. These findings suggest that patient survival in C-SCLC may be mainly based on the histological components, stage, and gene status of the tumor. In addition, active molecular detection and reasonable treatment may substantially increase patient survival.
The limitations of this study should be mentioned. First, due to the poor PS score and will of the patient, a histological specimen for assessing progression after third-line treatment was not collected. Therefore, the exact tumor genetic status before selpercatinib administration could not be determined. However, cfDNA extracted from blood serum for high-depth NGS after the third line of treatment showed a low level of the KIF5B-RET fusion, which may play an important role in patients who could not undergo histological biopsy. In addition, since the sample was obtained by biopsy, it was insufficient for fluorescence in situ hybridization (FISH), which could help determine the origin of the pathological type (SCLC or LCNEC) of the RET fusion.
Conclusion
In conclusion, we present the first report of a case of combined SCLC and LCNEC with RET fusion-positivity showing an ongoing treatment response to selpercatinib in 4th-line therapy for 14 months. Identification of actionable genomic alterations by molecular profiling may play an important role in the clinical management of C-SCLC. Selective RET inhibitors are a viable therapeutic option for RET fusion-driven C-SCLC.
Data Sharing Statement
All inquiries can be directed to the corresponding authors.
Ethics Statement and Consent for Publication
Institutional approval was not required to publish the case details. The patient provided written informed consent to the publication of this case report and any accompanying images.
Acknowledgment
The authors thank the patient and her family for their support of this study.
Funding
This work was supported by Sichuan University Innovation Research Project, Grant/Award Number: 2022SCUH0018, Sichuan University.
Disclosure
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
1. WHO Classification of Tumours Editorial Board. Thoracic Tumours. 5th ed. Lyon, France: International Agency for Research on Cancer; 2021.
2. Nicholson SA, Beasley MB, Brambilla E, et al. Small cell lung carcinoma (SCLC): a clinicopathologic study of 100 cases with surgical specimens. Am J Surg Pathol. 2002;26(9):1184–1197. doi:10.1097/00000478-200209000-00009
3. Qin J, Lu H. Combined small-cell lung carcinoma. Onco Targets Ther. 2018;19(11):3505–3511. doi:10.2147/OTT.S159057
4. National Comprehensive Cancer Network. Small Cell Lung Cancer (version 3.2023). Available from: https://www.nccn.org/professionals/physician_gls/pdf/sclc.pdf. Accessed November 27, 2023.
5. Kohno T, Tsuta K, Tsuchihara K, et al. RET fusion gene: translation to personalized lung cancer therapy. Cancer Sci. 2013;104(11):1396–1400. doi:10.1111/cas.12275
6. Subbiah V, Cote GJ. Advances in Targeting RET-Dependent Cancers. Cancer Discov. 2020;10(4):498–505. doi:10.1158/2159-8290.CD-19-1116
7. Drusbosky LM, Rodriguez E, Dawar R, et al. Therapeutic strategies in RET gene rearranged non-small cell lung cancer. J Hematol Oncol. 2021;14(1):50. doi:10.1186/s13045-021-01063-9
8. Wang R, Hu H, Pan Y, et al. RET fusions define a unique molecular and clinicopathologic subtype of non-small-cell lung cancer. J Clin Oncol. 2012;30(35):4352–4359. doi:10.1200/JCO.2012.44.1477
9. Drilon A, Lin JJ, Filleron T, et al. Frequency of brain metastases and multikinase inhibitor outcomes in patients with RET-rearranged lung cancers. J Thorac Oncol. 2018;13(10):1595–1601. doi:10.1016/j.jtho.2018.07.004
10. Shi M, Wang W, Zhang J, et al. Identification of RET fusions in a Chinese multicancer retrospective analysis by next-generation sequencing. Cancer Sci. 2022;113(1):308–318. doi:10.1111/cas.15181
11. Ignatius Ou S-H, Zhu VW. Catalog of 5’ fusion partners in RET+ NSCLC Circa 2020. JTO Clin Res Rep. 2020;1(2):100037. doi:10.1016/j.jtocrr.2020.100037
12. Feng J, Li Y, Wei B, et al. Clinicopathologic characteristics and diagnostic methods of RET rearrangement in Chinese non-small cell lung cancer patients. Transl Lung Cancer Res. 2022;11(4):617–631. doi:10.21037/tlcr-22-202
13. Larkin HD. Selpercatinib receives regular approval for non-small cell lung cancer. JAMA. 2022;328(17):1679. doi:10.1001/jama.2022.18445
14. Markham A. Pralsetinib: first Approval. Drugs. 2020;80(17):1865–1870. doi:10.1007/s40265-020-01427-4
15. Novello S, Califano R, Reinmuth N, et al. RET fusion-positive non-small cell lung cancer: the evolving treatment landscape. Oncologist. 2023;28(5):402–413. doi:10.1093/oncolo/oyac264
16. Li Y, Chen R, Yuan M, et al. One-stop molecular classification of endometrial carcinoma using comprehensive next-generation sequencing. Int J Cancer. 2022;151(11):1969–1977. doi:10.1002/ijc.34241
17. Zhao X, McCutcheon JN, Kallakury B, et al. Combined small cell carcinoma of the lung: is it a single entity? J Thorac Oncol. 2018;13(2):237–245. doi:10.1016/j.jtho.2017.10.010
18. Zhang J, Zhang L, Luo J, et al. Comprehensive genomic profiling of combined small cell lung cancer. Transl Lung Cancer Res. 2021;10(2):636–650. doi:10.21037/tlcr-20-1099
19. Simbolo M, Centonze G, Ali G, et al. Integrative molecular analysis of combined small-cell lung carcinomas identifies major subtypes with different therapeutic opportunities. ESMO Open. 2022;7(1):100308. doi:10.1016/j.esmoop.2021.100308
20. Niitsu T, Shiroyama T, Miyake K, et al. Combined small cell lung carcinoma harboring ALK rearrangement: a case report and literature review. Thorac Cancer. 2020;11(12):3625–3630. doi:10.1111/1759-7714.13716
21. Lu H, Xu H, Xie F, et al. 1p/19q codeletion and RET rearrangements in small-cell lung cancer. Onco Targets Ther. 2016;16(9):3571–3577. doi:10.2147/OTT.S108781
22. Mulligan LM, Timmer T, Ivanchuk SM, et al. Investigation of the genes for RET and its ligand complex, GDNF/GFR alpha-I, in small cell lung carcinoma. Genes Chromos Cancer. 1998;21(4):326–332. doi:10.1002/(SICI)1098-2264(199804)21:43.0.CO;2-0
23. Lou G, Yu X, Song Z. Molecular profiling and survival of completely resected primary pulmonary neuroendocrine carcinoma. Clin Lung Cancer. 2017;18(3):e197–e201. doi:10.1016/j.cllc.2016.11.014
24. Arora A, Zaemes J, Ozdemirli M, et al. Response to selpercatinib in a patient with RET fusion-positive pulmonary large-cell neuroendocrine carcinoma: a case report. Front Oncol. 2023;13:1134151. doi:10.3389/fonc.2023.1134151
25. Lei L, Chen Q, Wang Z, et al. Usefulness of carcinoembryonic antigen in the diagnosis of small cell lung cancer combined with adenocarcinoma. Adv Clin Exp Med. 2017;26(7):1091–1094. doi:10.17219/acem/66372
26. Subbiah V, Gainor JF, Oxnard GR, et al. Intracranial efficacy of selpercatinib in ret fusion-positive non-small cell lung cancers on the LIBRETTO-001 trial. Clin Cancer Res. 2021;27(15):4160–4167. doi:10.1158/1078-0432.CCR-21-0800
27. Drilon A, Subbiah V, Gautschi O, et al. Selpercatinib in patients with RET fusion-positive non-small-cell lung cancer: updated safety and efficacy from the registrational LIBRETTO-001 phase I/II trial. J Clin Oncol. 2023;41(2):385–394. doi:10.1200/JCO.22.00393
28. Illini O, Hochmair MJ, Fabikan H, et al. Selpercatinib in RET fusion-positive non-small-cell lung cancer (SIREN): a retrospective analysis of patients treated through an access program. Ther Adv Med Oncol. 2021;11(13):17588359211019675. doi:10.1177/17588359211019675
29. Babakoohi S, Fu P, Yang M, et al. Combined SCLC clinical and pathologic characteristics. Clin Lung Cancer. 2013;14(2):113–119. doi:10.1016/j.cllc.2012.07.002
30. Yang L, Zhou Y, Wang G, et al. Clinical features and prognostic factors of combined small cell lung cancer: development and validation of a nomogram based on the SEER database. Transl Lung Cancer Res. 2021;10(11):4250–4265. doi:10.21037/tlcr-21-804