Cancer and neoplasms
Systemic inflammatory indices for predicting prognosis of myelofibrosis
Abstract
The impact of inflammatory markers such as systemic immune-inflammation (SII) index and systemic inflammation response index (SIRI) on myelofibrosis (MF) prognosis was evaluated for the first time in this study. Data from 60 patients diagnosed with MF between March 2011 and September 2022 were retrospectively analyzed. In addition to disease-related markers, the impact of SII and SIRI on prognosis was evaluated. In our study, the overall median survival (OS) was 64 months. OS was significantly shorter in patients older than 65 years, with high ferritin and lymphocyte levels, transfusion dependence at diagnosis, platelet count below 100 × 109/L, Hb level below 8 g/dl, and high risk according to the dynamic international prognostic scoring system (DIPSS)-Plus score. When these variables were included in the multivariate Cox regression model, it was found that being older than 65 years, having a high ferritin value, being at high risk according to the DIPSS-plus score and Hb values below 8 increased the risk of death. Platelet-to-lymphocyte ratio (PLR) and SII index were lower in patients with a fatal outcome. No statistically significant relationship was found between SIRI and mortality. The findings of this study showed that low PLR and high ferritin were associated with poor prognosis in MF. Elevated SII and SIRI, evaluated for the first time in patients with myelofibrosis, did not predict prognosis. Since non-inflammatory variables play a role in the pathogenesis of MF, bone marrow indicators and systemic inflammation indicators derived from hematologic parameters may not be accurate.
Introduction
Myelofibrosis (MF) is a BCR-ABL1-negative myeloproliferative neoplasms (MPN) characterized by anemia, extramedullary hematopoiesis, bone marrow fibrosis, splenomegaly, constitutional symptoms, and acute myeloid leukemia progression1. Most patients carry a mutation in the JAK-2, CALR, or MPL genes2, which contributes to the JAK-2-signal-transducer-and-activator-of-transcription signaling pathway and the high inflammatory state characteristic of these diseases. Inflammation plays a crucial role in the development and progression of MPN.
The prognosis of MF varies greatly. While some patients only have a few months to survive, others live for more than 20 years. The three leading causes of death are hemorrhage, infection brought on by bone marrow loss, and transformation into acute leukemia3.
A good risk stratification model provides information about the prognosis of patients, affecting the decision whether the patient is included as a candidate for allogeneic stem cell transplantation and thereby the treatment. To evaluate the mortality risk of MF patients, the dynamic international prognostic scoring system (DIPSS)4 or DIPSS-plus is generally used5.
It is well established that inflammation affects all phases of tumor growth and can increase the risk of developing a tumor (triggering the first genetic mutation, tumor development, metastasis, and progression). Therefore, inflammation parameters are strong candidates for predicting cancer prognosis. Numerous inflammatory indicators have recently been linked to a poor prognosis for cancer, including C-reaction protein (CRP), neutrophil-to-lymphocyte ratio (NLR), and platelet-to-lymphocyte ratio (PLR)6,7,8. The systemic immune-inflammation (SII), which was first used in hepatocellular cancer in 2014 as a new inflammation marker based on peripheral neutrophil, platelet, and lymphocyte counts9 and the systemic inflammation response index (SIRI), which was developed to predict survival in patients with pancreatic cancer and based on peripheral neutrophil, monocyte, and lymphocyte counts10, have also been used as inflammatory biomarkers to predict prognosis in many cancer types11,12,13,14.
In this study, in addition to the potential prognostic markers examined in other retrospective studies to date, we investigated the prognostic value of SII and SIRI in MF for the first time in the literature.
Materials and methods
The data of 60 patients who were followed up with the diagnosis of MF between March 2011 and September 2022 at Bursa Uludag University Hematology Department Clinic were retrospectively analyzed. All patients met the 2016 World Health Organization criteria for PMF15 or the 2008 international working group for myelofibrosis research and treatment (IWG-MRT) criteria for SMF16. Patients were divided into four groups as low, intermediate-1, intermediate-2, and high-risk groups according to DIPSS and DIPSS-plus scores. The effect of age, MF subtype, JAK-2 mutation status, erythrocyte transfusion dependence, presence of constitutional symptoms, splenomegaly, leukocyte, hemoglobin, platelet, CRP, mean corpuscular volume, mean platelet volume (MPV), red blood cell distribution width (RDW), LDH, ferritin levels, TS and CAR, PNI, NLR, PLR, leukocyte to lymphocyte ratio (WLR), ferritin to lymphocyte ratio (FLR), lymphocyte to LDH ratio (LLR), DIPSS and DIPSS-plus risk group, and SII and SIRI, which were examined for the first time in MF, on prognosis was investigated in all patients. SII was calculated as platelet count × neutrophil count/lymphocyte count in peripheral blood and SIRI was calculated as neutrophil count × monocyte count/lymphocyte count. Mortality was defined as patients who died during follow-up. Our study was conducted under the institutional research committee’s ethical standards and according to the 1964 Helsinki Declaration. This study was approved by the clinical research ethics committee of Bursa Uludag University Faculty of Medicine (Decision No: 2022-18/20).
Statistical analysis
Statistical analyses were performed using IBM SPSS Statistics for Windows Version 25.0 (Statistical Package for the Social Sciences, IBM Corp., Armonk, NY, USA). Descriptive statistics were presented as n and % for categorical variables and mean ± SD, median (IQR) for continuous variables. Data were analyzed in terms of normality assumptions. For continuous variables with Kolmogorov–Smirnov values p > 0.05, independent samples t-test was used as the parametric test to evaluate the difference in mortality between the groups. Chi-square test was used to compare categorical variables. Three receiver operating characteristic (ROC) curve analysis was performed for SII and ferritin values to predict mortality. Kaplan–Meier method was used to compare survival times between various variables. Finally, multivariate Cox regression results of various clinical factors on mortality risk were presented. p < 0.05 was considered statistically significant in all analyses.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent: Informed consent was obtained from all individual participants included in the study.
Results
Sixty patients made up the study population, with 53.3% of the females and 46.7% of the males. Median age at diagnosis of 63 years. Most patients (76.6%) and those with splenomegaly (93.3%) had anemia at the time of diagnosis. Of these patients, 41.6% had massive splenomegaly. There were 41.6% of cases of constitutional symptoms, with weight loss being the most prevalent (61.5%) and high fever being the least prevalent (11.5%). Thrombosis was present in 16.6% of patients (n = 10). Four patients had cerebral vascular thrombosis, five had portal system thrombosis (three portal vein thrombosis, two splenic infarction), and one had deep vein thrombosis. JAK-2 mutation positivity was detected in 30 (58.8%) of 51 patients who underwent genetic screening. The rate of transformation to acute leukemia was 8.3%. Table 1 shows the distribution of the demographic and clinical findings of the patients.
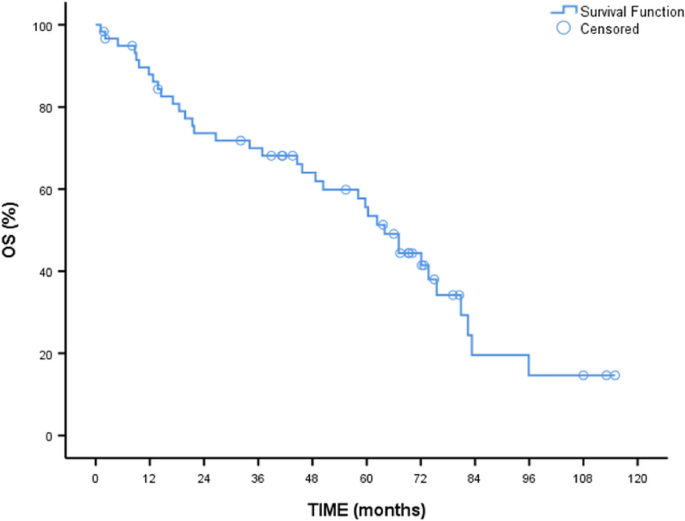
As seen in Table 2, median overall median survival (OS) was 64 months (95% CI 54.88–73.10). Two-year OS was 73.6%, while five-year OS was 55.6%. No difference was found between the MF subtypes (p = 0.825). Median OS was significantly different between the age groups (p = 0.005) (Fig. 1). Median survival was 73.7 months in patients under 65 years of age (95% CI 68.07–79.32) compared to 44.6 months in patients over 65 years of age (95% CI 10.66–78.53). Two-year OS and five-year OS were 83.3% and 74.1% in patients under 65 years of age compared with 56.4% and 25.6% in patients over 65 years of age.
Overall survival of patients.
A significant difference was found in OS with respect to DIPSS-plus risk groups (p < 0.001). Median survival was 73.7 months (95% CI 47.43–99.66) in the low-risk group, 72.1 months (95% CI –) in the intermediate-1 risk group, 59.7 months (95% CI 31.17–82.22) in the intermediate-2 risk group, and 9.6 months in the high-risk group (95% CI 8.06–11.4). A statistically significant difference was also found in median OS between the high-risk group and all other risk groups (p < 0.001). Two-year OS and five-year OS were 100% and 83.3% in the low-risk group, compared with 92.3% and 84.6% in the intermediate-1 risk group. In the intermediate-2 risk group, two-year OS was 76.3% and five-year OS was 49.9%. In the high-risk group, all patients died within two years.
PLR (p = 0.048), SII (p = 0.018) and lymphocyte count (p = 0.033) showed a statistically significant difference between patients with and without mortality. PLR and SII were lower in patients with mortality compared to patients without mortality, while ferritin and lymphocyte levels were higher. No significant difference was found in survival with respect to other variables (neutrophils, platelets, NLR, LLR, WLR, FLR, RDW, MPV, CRP, LDH, spleen size, serum iron, iron binding capacity, TS, PNI, CAR).
A significant relationship was found between OS and Hb levels below 8 g/dL (p = 0.027), transfusion dependency (p < 0.001), and platelet count below 100 × 109/L (p = 0.002), while no statistically significant relationship was found between OS and MF subtypes, positive or negative JAK-2 mutation status, presence of constitutional symptoms, TS < 20%, Hb 25 × 109/L, degree of collagen and reticulin fibrosis, and DIPSS risk groups.
Univariate analysis results showed that age, ferritin, transfusion dependency, platelet count, DIPSS-plus risk group, Hb, and SII index variables were statistically significant for mortality risk (p < 0.05). The variables that were significant in univariate analyses were included in the multivariate Cox regression model. According to the results of the multivariate Cox regression model, it was found that being over 65 years of age (HR 7.29; 95% CI 2.44–21.75; p < 0.001), increased ferritin values (HR 1.00; 95% CI 1.00–1.01 p = 0.002), high-risk DIPSS-plus (HR 12.63; 95% CI 1.30–122.30 p = 0.029), and hemoglobin values below 8 increased mortality risk (OR 0.32; 95% CI 0.11–0.94 p = 0.038) (p < 0.001, − 2 loglikelihood = 158,326) (Table 3).
The predictive power of SII for mortality was statistically significant (p = 0.032). In the ROC analysis conducted to evaluate SII in predicting mortality, the area under the curve was 0.677 (95% CI 0.528–0.827) and the cut-off value for SII was 1246.78 (Fig. 2). For this value and below, sensitivity was 57.6% and specificity was 55.5%. The predictive power of ferritin was not statistically significant for mortality (p = 0.097) (Table 4, Fig. 3).

ROC curve of SII levels for predicting mortality.

ROC curve of Ferritin levels for predicting mortality.
Discussion
Accurate risk assessment is crucial for developing the best treatment strategy in MF, which is one of the BCR-ABL-negative MPNs accepted as a model of inflammation-related cancer development, especially in young patients. Widely accepted scoring systems require genetic evaluation and tests may be difficult to access in some centers. The current study assessed prognostic indicators in patients with MF. To the best of our knowledge, this is the first study to have examined the relationship between SII and SIRI and mortality in MF patients in the literature. Inflammatory indicators and parameters that could affect prognosis were assessed in 60 patients.
Indices such as SII and SIRI are thought to be associated with the prognosis of various tumors. A meta-analysis by Yang et al. evaluating 22 studies including 7657 patients revealed that high SII was clearly associated with lower OS, time to recurrence, progression-free survival, cancer-specific survival, relapse-free survival, and disease-free survival17. These results suggest that high SII may be a potential prognostic marker in patients with various cancers and may be associated with poor overall outcomes. A study by Geng et al. in patients with esophageal cancer showed that median OS was significantly higher in patients with low SIRI18.
In this study, the relationship between MF and SII was evaluated for the first time in the literature; and paradoxically, mortality was found to be lower in patients with MF compared to patients without MF (p = 0.018). This discrepancy results from patients with a fatal course having higher lymphocyte numbers and lower platelet counts. SII lost its relevance when these variables were incorporated into the multivariate Cox regression model. Additionally, SIRI was also examined for the first time in MF patients and found not to be associated with mortality (p = 0.492).
Anemia is a disease characteristic most consistently associated with poor prognosis in MF5,19,20,21. The most commonly used threshold in prognostic models is 10 g/dL. Transfusion dependence has had poor prognostic significance in MF22,23,24. There is ongoing debate about the relationship between transfusion dependence and poor prognosis in chronic MPN. Some authors argue that transfusion dependence affects survival through the adverse effects of chronic erythrocyte transfusion, such as iron overload and transfusion-related immunomodulation. In the present study, when the Hb cut-off point was taken as 10 g/dL, no difference was found between the groups in terms of survival (p = 0.168). However, when the cut-off point was taken as 8 g/dL, a significant difference was found in median OS (p < 0.027). Hb level ≤ 8 g/dL was determined as a marker of poor prognosis. When included in the multivariate Cox regression model, Hb < 8 g/dL increases the risk of mortality (HR 0.32; 95% CI 0.11–0.94 p = 0.038).
Some studies have found that thrombocytopenia was associated with poor prognosis5,20,21,25, but it was noted that low platelet counts are frequently associated with anemia and collinearity in multivariate regression models may make it difficult to characterize thrombocytopenia as an independent prognostic factor19. In the present study, platelet count was lower in patients with a mortal course, but the difference was not statistically significant (p = 0.085). When the cut-off value for platelet count was taken as 100 × 109/L, a significant difference was found in median OS (p = 0.002). Median OS was significantly shorter in patients with platelet count below 100 × 109/L (72.1 months versus 17.1 months).
The transfusion dependency at diagnosis or during MF is an indicator of poor prognosis22,23. In our patients, median OS was 73.7 months in the group without transfusion dependence and 17.1 months in the group with transfusion dependence, which was significantly lower (p < 0.001).
Consistent with previous studies, age was associated with OS in both univariate analysis and multivariate Cox regression analysis. When the cut-off point for age was taken as 65 years, a significant difference was found in OS between the groups (p = 0.005). When included in the multivariate Cox regression model, it was found that mortality risk was significantly higher in those older than 65 years (HR 7.29; 95% CI 2.44–21.75; p < 0.001).
Currently, the most widely used prognostic scoring system in MF is DIPSS-plus. DIPSS-plus was used in 967 consecutive patients at the Mayo Clinic and resulted in median survival of 1.8, 3.6, 7.8, and 17.5 years for high, intermediate-2, intermediate-1, and low-risk patients, respectively26. When the patients in this study were divided into risk groups according to DIPSS-plus, a statistically significant difference was found between the median OS times (p < 0.001). Median OS was 73.7 months in the low-risk group, 72.1 months in the intermediate-1 risk group, 59.7 months in the intermediate-2 risk group, whereas it was 9.6 months in the high-risk group (p < 0.001). In the multivariate Cox regression analysis, a significant difference was found between the low-risk group and the high-risk group persisted (HR 12.63; 95% CI 1.30–122.30 p = 0.029), while the significance between the low-risk group and intermediate-1 and intermediate-2 risk groups disappeared (p = 0.151, p = 0.570, respectively).
With respect to iron metabolism, studies have shown that high ferritin value and low TS are associated with low OS in MF. Lucijanic et al. evaluated the prognostic impact of low TS in 87 patients with PMF. Low TS was found to have a detrimental effect on the survival of PMF patients, independent of anemia and ferritin levels27. In the present study, ferritin level was found to be higher in patients with mortality (p = 0.024) and when included in the multivariate Cox regression model, it was found that an increase in ferritin levels increased the risk of mortality (HR 1.00; 95% CI 1.00–1.01 p = 0.002). However, there was no statistically significant relationship between TS, serum iron level, iron binding capacity, RDW, and mortality. Numerous studies have been published in the literature showing the link between several inflammation markers, including NLR and PLR, and a poor prognosis for cancer7,8,28. In a study evaluating NLR and PLR in MF, these values were found to be significantly higher in patients compared to healthy controls. In univariate analyses, shorter overall survival was observed in patients presenting with high NLR and low PLR29. In the same study, increased RDW was associated with survival (p = 0.039). In MF, high CRP is associated with features of more advanced disease and a trend toward worse clinical outcomes as part of individual parameters or different prognostic scores30,31,32. In this study, no correlation was found between CRP and NLR and survival. Consistent with the literature, survival was shorter in patients with low PLR values (p = 0.048).
CAR has recently been recognized as an inflammatory biomarker and prognostic factor in several malignant neoplasms33,34. However, a study evaluating CAR in patients with MF reported that higher CAR was associated with lower OS35. PNI is an index reflecting a patient’s inflammatory, nutritional, and immune status. In a study evaluating PNI in MF patients, low PNI predicted worse survival independent of DIPSS36. In the present study, however, CAR and PNI had no effect on survival.
The mean LDH level was higher (p = 0.108) and spleen size was larger (p = 0.122) in patients with a mortal outcome, but this was not statistically significant.
This study has certain limitations. The study was conducted retrospectively and in a single-center.
In conclusion, the results obtained in this study show that elevated SII and SIRI, which have prognostic significance for many cancers, cannot be used as markers for poor prognosis in MF. Since the pathology of MF directly involves the bone marrow unlike solid organ cancers, these inflammation markers may be insufficient to predict prognosis. Further clinical studies are needed to confirm these results.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
-
Tefferi, A. & Barbui, T. Polycythemia vera and essential thrombocythemia: 2019 update on diagnosis, risk-stratification and management. Am. J. Hematol. 94(1), 133–143. https://doi.org/10.1002/ajh.25303 (2019).
Google Scholar
-
Tefferi, A. et al. CALR vs JAK2 vs MPL-mutated or triple-negative myelofibrosis: Clinical, cytogenetic and molecular comparisons. Leukemia 28(7), 1472–1477. https://doi.org/10.1038/leu.2014.3 (2014).
Google Scholar
-
Campanelli, R., Massa, M., Rosti, V. & Barosi, G. New markers of disease progression in myelofibrosis. Cancers 13(21), 5324. https://doi.org/10.3390/cancers13215324 (2021).
Google Scholar
-
Passamonti, F. et al. A dynamic prognostic model to predict survival in primary myelofibrosis: A study by the IWG-MRT (international working group for myeloproliferative neoplasms research and treatment). Blood 115(9), 1703–1708. https://doi.org/10.1182/blood-2009-09-245837 (2010).
Google Scholar
-
Gangat, N. et al. DIPSS plus: A refined dynamic international prognostic scoring system for primary myelofibrosis that incorporates prognostic information from karyotype, platelet count, and transfusion status. J Clin. Oncol. 29(4), 392–397. https://doi.org/10.1200/jco.2010.32.2446 (2011).
Google Scholar
-
Huang, Q. T. et al. Prognostic significance of neutrophil-to-lymphocyte ratio in ovarian cancer: A systematic review and meta-analysis of observational studies. Cell Physiol. Biochem. 41(6), 2411–2418. https://doi.org/10.1159/000475911 (2017).
Google Scholar
-
Cummings, M. et al. Preoperative neutrophil:lymphocyte and platelet:lymphocyte ratios predict endometrial cancer survival. Br. J. Cancer. 113(2), 311–320. https://doi.org/10.1038/bjc.2015.200 (2015).
Google Scholar
-
Zheng, Z. et al. Prognostic role of C-reactive protein in hepatocellular carcinoma: A systematic review and meta-analysis. Int. J. Med. Sci. 10(6), 653–664. https://doi.org/10.7150/ijms.6050 (2013).
Google Scholar
-
Hu, B. et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin. Cancer Res. 20(23), 6212–6222. https://doi.org/10.1158/1078-0432.Ccr-14-0442 (2014).
Google Scholar
-
Qi, Q. et al. A novel systemic inflammation response index (SIRI) for predicting the survival of patients with pancreatic cancer after chemotherapy. Cancer 122(14), 2158–2167. https://doi.org/10.1002/cncr.30057 (2016).
Google Scholar
-
Sun, Y. et al. Increased systemic immune-inflammation index independently predicts poor survival for hormone receptor-negative, HER2-positive breast cancer patients. Cancer Manag. Res. 11, 3153–3162. https://doi.org/10.2147/cmar.S190335 (2019).
Google Scholar
-
Huang, H. et al. Prognostic value of preoperative systemic immune-inflammation index in patients with cervical cancer. Sci. Rep. https://doi.org/10.1038/s41598-019-39150-0 (2019).
Google Scholar
-
Zhu, M. et al. The Systemic inflammation response index as an independent predictor of survival in breast cancer patients: A retrospective study. Front. Mol. Biosci. 9, 856064. https://doi.org/10.3389/fmolb.2022.856064 (2022).
Google Scholar
-
Chen, L. et al. In gastric cancer patients receiving neoadjuvant chemotherapy systemic inflammation response index is a useful prognostic indicator. Pathol. Oncol. Res. 27, 1609811. https://doi.org/10.3389/pore.2021.1609811 (2021).
Google Scholar
-
Arber, D. A. et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 127(20), 2391–2405. https://doi.org/10.1182/blood-2016-03-643544 (2016).
Google Scholar
-
Barosi, G. et al. Proposed criteria for the diagnosis of post-polycythemia vera and post-essential thrombocythemia myelofibrosis: A consensus statement from the international working group for myelofibrosis research and treatment. Leukemia 22(2), 437–438. https://doi.org/10.1038/sj.leu.2404914 (2008).
Google Scholar
-
Yang, R., Chang, Q., Meng, X., Gao, N. & Wang, W. Prognostic value of Systemic immune-inflammation index in cancer: A meta-analysis. J. Cancer. 9(18), 3295–3302. https://doi.org/10.7150/jca.25691 (2018).
Google Scholar
-
Geng, Y. et al. A novel systemic inflammation response index (SIRI) for predicting postoperative survival of patients with esophageal squamous cell carcinoma. Int. Immunopharmacol. 65, 503–510. https://doi.org/10.1016/j.intimp.2018.10.002 (2018).
Google Scholar
-
Cervantes, F. et al. New prognostic scoring system for primary myelofibrosis based on a study of the international working group for myelofibrosis research and treatment. Blood 113(13), 2895–2901. https://doi.org/10.1182/blood-2008-07-170449 (2009).
Google Scholar
-
Tefferi, A. et al. Validation and comparison of contemporary prognostic models in primary myelofibrosis. Cancer 109(10), 2083–2088. https://doi.org/10.1002/cncr.22630 (2007).
Google Scholar
-
Morel, P. et al. Identification during the follow-up of time-dependent prognostic factors for the competing risks of death and blast phase in primary myelofibrosis: A study of 172 patients. Blood 115(22), 4350–4355. https://doi.org/10.1182/blood-2009-12-255943 (2010).
Google Scholar
-
Tefferi, A. et al. Red blood cell transfusion need at diagnosis adversely affects survival in primary myelofibrosis-increased serum ferritin or transfusion load does not. Am. J. Hematol. 84(5), 265–267. https://doi.org/10.1002/ajh.21391 (2009).
Google Scholar
-
Tefferi, A. et al. Transfusion- dependency at presentation and its acquisition in the first year of diagnosis are both equally detrimental for survival in primary myelofibrosis–prognostic relevance is independent of IPSS or karyotype. Am. J. Hematol. 85(1), 14–17. https://doi.org/10.1002/ajh.21574 (2010).
Google Scholar
-
Elena, C. et al. Red blood cell transfusion-dependency implies a poor survival in primary myelofibrosis irrespective of IPSS and DIPSS. Haematologica 96(1), 167–170. https://doi.org/10.3324/haematol.2010.031831 (2011).
Google Scholar
-
Reilly, J. T. et al. Cytogenetic abnormalities and their prognostic significance in idiopathic myelofibrosis: A study of 106 cases. Br. J. Haematol. 98(1), 96–102. https://doi.org/10.1046/j.1365-2141.1997.1722990.x (1997).
Google Scholar
-
Tefferi, A. et al. One thousand patients with primary myelofibrosis: The mayo clinic experience. Mayo Clin. Proc. 87(1), 25–33. https://doi.org/10.1016/j.mayocp.2011.11.001 (2012).
Google Scholar
-
Lucijanic, M. et al. Prognostic implications of low transferrin saturation in patients with primary myelofibrosis. Leuk. Res. 66, 89–95. https://doi.org/10.1016/j.leukres.2018.01.017 (2018).
Google Scholar
-
Huang, H. et al. Prognostic value of preoperative systemic immune-inflammation index in patients with cervical cancer. Sci. Rep. 9(1), 3284. https://doi.org/10.1038/s41598-019-39150-0 (2019).
Google Scholar
-
Lucijanic, M. et al. Elevated Neutrophil–to–lymphocyte-ratio and platelet–to–lymphocyte ratio in myelofibrosis: Inflammatory biomarkers or representatives of myeloproliferation itself?. Anticancer Res. 38(5), 3157–3163 (2018).
Google Scholar
-
Barbui, T. et al. Elevated C-reactive protein is associated with shortened leukemia-free survival in patients with myelofibrosis. Leukemia 27(10), 2084–2086. https://doi.org/10.1038/leu.2013.207 (2013).
Google Scholar
-
Barosi, G. et al. Primary myelofibrosis: Older age and high JAK2V617F allele burden are associated with elevated plasma high-sensitivity C-reactive protein levels and a phenotype of progressive disease. Leuk. Res. 60, 18–23. https://doi.org/10.1016/j.leukres.2017.06.004 (2017).
Google Scholar
-
Lucijanic, M. et al. Combining information on C reactive protein and serum albumin into the Glasgow prognostic score strongly discriminates survival of myelofibrosis patients. Blood Cells Mol. Dis. 72, 14–16. https://doi.org/10.1016/j.bcmd.2018.06.001 (2018).
Google Scholar
-
Shibutani, M. et al. Prognostic significance of the C-reactive protein-to-albumin ratio in patients with metastatic colorectal cancer treated with trifluridine/thymidine phosphorylase inhibitor as later-line chemotherapy. Anticancer Res. 39(2), 1051–1057. https://doi.org/10.21873/anticanres.13212 (2019).
Google Scholar
-
Li, Y. J. et al. Clinical implications of six inflammatory biomarkers as prognostic indicators in Ewing sarcoma. Cancer Manag. Res. 9, 443–451. https://doi.org/10.2147/cmar.S146827 (2017).
Google Scholar
-
Lucijanic, M. et al. C reactive protein to albumin ratio as prognostic marker in primary and secondary myelofibrosis. Leuk. Lymphoma. 61(12), 2969–2974. https://doi.org/10.1080/10428194.2020.1789627 (2020).
Google Scholar
-
Lucijanic, M. et al. Assessing serum albumin concentration, lymphocyte count and prognostic nutritional index might improve prognostication in patients with myelofibrosis. Wien. Klin. Wochenschr. 130(3–4), 126–133. https://doi.org/10.1007/s00508-018-1318-z (2018).
Google Scholar
Author information
Authors and Affiliations
Contributions
T.E. and F.Ö. wrote the main manuscript. B.O,. C.Y., Ö.C, F.Ç.H. and Ş.Y contributed planning methodology, preparing tables and figures. İ.E.P, S.Ç., T.G.K contributed to data collection and data management. V.Ö and R.A. reviewed the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
Reprints and Permissions
About this article
Cite this article
Ersal, T., Özkocaman, V., Pınar, İ.E. et al. Systemic inflammatory indices for predicting prognosis of myelofibrosis.
Sci Rep 13, 12539 (2023). https://doi.org/10.1038/s41598-023-39077-7
-
Received: 17 March 2023
-
Accepted: 19 July 2023
-
Published: 02 August 2023
-
DOI: https://doi.org/10.1038/s41598-023-39077-7
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.