Congenital disorders
Chronic hypertension in pregnancy
Chronic hypertension (CHTN) is a condition in which elevated blood pressure (BP) predates pregnancy or is first diagnosed in pregnancy before 20 weeks’ gestation.1,2 It affects approximately 2% or more of pregnant patients, depending on diagnostic criteria,3,4 and is associated with increased short- and longterm adverse maternal and neonatal outcomes.1,2,5-9 The American College of Obstetricians and Gynecologists (ACOG) defines CHTN during pregnancy as elevated BPs greater than 140/90 mm Hg on at least 2 occasions, 4 hours apart, and occurring before 20 weeks’ gestation.2 However, in the nonpregnant patient, the diagnosis of CHTN is now ≥130/80 mm Hg on at least 2 occasions at least 4 hours apart, following recent changes to diagnosis guidelines in 2017 by the American College of Cardiology(ACC) and the American Heart Association (AHA).10,11 These changes in diagnostic criteria were a result of the increased long-term cardiovascular morbidity in individuals with BPs in the range of 130- to 139/80- to 89 mm Hg. Therefore, nonpregnant patients will be diagnosed with CHTN using lower BP thresholds than those used in pregnancy and will in turn increase the prevalence of pregnant patients with CHTN.2
Diagnosis and classification
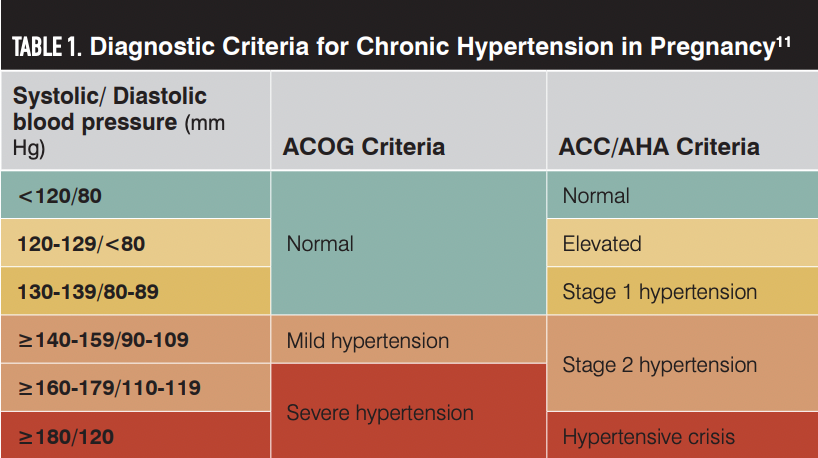
The AHA/ACC guidelines classify CHTN into normal, elevated, stage 1 hypertension, stage 2 hypertension, or hypertensive crisis categories based on BP levels (Table 1).11 Hypertension in pregnancy is classified as severe (BP ≥ 160/110 mm Hg) or mild (BP, 140-159/90-109 mm Hg) by ACOG. Classification of hypertension correlates with the degree of maternal and fetal risks and identifies patients who need acute therapy to lower BPs during pregnancy.12 Accurate measurement of BP is crucial to correctly identify patients with CHTN during pregnancy (Box).13,14
ACC, American College of Cardiology; ACOG, American College of Obstetricians and Gynecologists; AHA, American Heart Association.
Risk factors and adverse outcomes
Nonmodifiable risk factors for CHTN include increasing age, Black race, and family history of CHTN. Modifiable risk factors include obesity (approximately 40% of reproductive-aged individuals in the United States),15 excess alcohol and tobacco consumption, physical inactivity, diabetes mellitus, chronic kidney disease, and diet that is high in sodium and cholesterol. CHTN is associated with short- and long-term risks. In nonpregnant adults with CHTN, compared with individuals with normal BPs, the risks of myocardial infarction, heart failure, chronic kidney disease, stroke, vision loss, and sexual dysfunction are increased.16-20 Similarly, during pregnancy, patients with CHTN have a 2-fold higher risk of maternal mortality, a 3-fold higher risk of cerebrovascular accidents and pulmonary edema, and up to a 16-fold higher risk of renal failure.13,21

Preeclampsia, a leading indication for indicated preterm delivery that occurs in 25% to 50% of patients with CHTN, is a leading mediator of adverse outcomes. Additionally, gestational diabetes and cesarean deliveries are also more frequent in patients with CHTN.2 The fetal risks of CHTN includes a 2- to 3-fold higher risk of low birth weight, preterm delivery, placental abruption, and stillbirth (Table 2).2,13,22,23 There are also increased risks of additional cardiovascular disease in later life in both individuals with CHTN and their off-spring (including hypertension, stroke, obesity, and cardiovascular disease) conceived in pregnancies complicated by CHTN or preeclampsia.13,24,25
SGA, small for gestational age.

Prevention
CHTN Lifestyle modification of risk factors remains the initial approach to CHTN prevention (Table 3). Gradual weight loss for overweight or obese patients26; cessation of tobacco use; regular moderate exercise; reduction in consumption of high-calorie, nutrient poor foods; and limitation of alcohol consumption27,28 are frequently cited effective strategies in nonpregnant patients. Alcohol consumption, even in small to moderate quantities, is not recommended in pregnancy or in the preconception period.
SBP, severe blood pressure.

Treatment
Prior to pregnancy, the lifestyle modification measures outlined above have been shown to reduce systolic BPs in patients with CHTN, with greater reductions when multiple measures are combined. Lifestyle measures also augment medication effectiveness in lowering BP, particularly in patients with drug-resistant hypertension. Bariatric surgery is recommended for patients with class 3 obesity (body mass index [BMI] ≥ 40 kg/m2 ) or who have a BMI greater than or equal to 35 kg/m2 and an obesity-related comorbidity.29 Pharmacologic BP reduction is associated with improved cardiovascular outcomes.30,31 The decision to initiate pharmacologic treatment in nonpregnant adults aged at least 40 years depends on the stage of CHTN and estimated atherosclerotic cardiovascular disease (ASCVD) risk, taking into consideration personal and family history. Pharmacologic treatment is generally recommended for ACC/AHA stage 2 CHTN, and for patients with ACC/AHA stage 1 hypertension with an ASCVD risk greater than 10% (Figure 1). In most patients, treatment goals are a systolic and diastolic BP of less than 130/80 mm Hg outside of pregnancy. However, in pregnancy, current evidence supports treatment to less than 140/90 mm Hg, although secondary analyses have reported improved outcomes with BPs at less than 130/80 mm Hg compared with less than 140/90 mm Hg.32,33 This remains an active area of investigation. However, another important note is that intensive treatment to lower BP goals in nonpregnant patients reduced adverse cardiovascular events in a clinical trial and was associated with significant adverse effects in a small subset of patients.35,36
FIGURE 1. Treatment Goals for Chronic Hypertension in Nonpregnant Patients.
ASCVD, atherosclerotic cardiovascular disease; BP, blood pressure; CVD, cardiovascular disease. Adapted from ACC/AHA Treatment of Hypertension 2017 Guidelines.

Angiotensin receptor blockers (ARBs), angiotensin-converting enzyme inhibitors (ACEIs), calcium channel blockers, or thiazide diuretics are the recommended first-line medications for treatment of CHTN in nonpregnant patients.36
Preconception and during pregnancy The ACC/AHA 2017 guidelines addressed first-line medications in pregnancy and medications to avoid but not treatment thresholds and goals. In the preconception period, antihypertensive agents including ARBs and ACEIs are associated with fetal harm (eg, oligohydramnios, renal failure, growth restriction, and bone abnormalities), especially with second- and third-trimester exposure, and should be discontinued. Patients should then be transitioned to labetalol or nifedipine.37 Although data are limited, patients who become pregnant while using alternate calcium channel blockers (such as amlodipine) with well-controlled BPs, or their physicians managing them, may choose to continue rather than switch to nifedipine during pregnancy.
Guidance regarding treatment thresholds and goals in pregnancy are provided by ACOG.2,32 Treatment was previously recommended only for severe hypertension (systolic blood pressure [SBP] ≥160 or diastolic blood pressure [DBP] ≥110 mm Hg) but not mild CHTN due to limited data regarding benefits and concerns that lowering BP beyond a certain threshold will lower placental perfusion, which in turn may lead to fetal growth restriction, as well as older studies reporting such associations.38-40 More recently, the Control of Hypertension in Pregnancy Study (CHIPS) study (NCT01192412), a multicountry trial of 987 women (75% with CHTN) found that tight control (target DBP of 85 mm Hg) compared with less tight (target DBP of 100 mm Hg) was not associated with an increased risk of pregnancy loss or high-level neonatal care more than 48 hours in the first 28 days of life (adjusted odds ratio [aOR], 1.02; 95% CI, 0.77-1.35) or maternal complications. However, severe hypertension was more prevalent in the less-tight group.40
Subsequently, the CHAP trial (NCT02299414) was published, which was a US-based, multicenter, pragmatic, open-label, randomized, controlled trial that focused on pregnant patients with mild CHTN (n = 2408). Patients were assigned to active treatment with a goal to reach BP of less than 140/90 mm Hg or standard care (no treatment unless BP ≥ 160/110 mm Hg). The rate of a composite primary outcome (1 or more of severe preeclampsia, medically indicated births prior to 35 weeks’ gestation, abruption, and fetal or neonatal death) was 18% lower in patients randomly assigned to active treatment to BP of less than 140/90 mm Hg (Figure 2).33 This was achieved without any significant difference in the risk of small for gestational age infants. In addition, other outcomes, including any preeclampsia, preterm birth, and low birth weight, were less frequent in the active group. Following publication, professional societies in the United States, including ACOG and the Society for Maternal-Fetal Medicine, have issued statements recommending patients with mild CHTN during pregnancy be treated to BP goals of less than 140/90 mm Hg.33,39
FIGURE 2. Outcomes of Treatment of Mild Chronic Hypertension During Pregnancy34
wga, weeks’ gestational age.

For patients with ACC/AHA stage 1 hypertension during pregnancy, the data are extremely limited on whether antihypertensive treatment or even prophylaxis with aspirin reduces the risks of adverse pregnancy outcomes.41,42 Thus, no firm recommendations can be made for this growing subset of pregnant patients other than lifestyle measures in pregnancy. Also, whether to treat CHTN to a goal of BP less than 130/80 mm Hg to align with recommendations outside of pregnancy vs BP of 140/90 mmHg after CHAP remains uncertain. These are areas for future investigation, given that stage 1 hypertension is associated with worse adverse outcomes
Nifedipine and labetalol are 2 recommended oral first-line agents with established safety profiles for the treatment of CHTN in pregnancy, but the preferred medication is not known.2 In a Cochrane review of randomized trials of treatment for mild to moderate CHTN in pregnancy, although β-blockers and calcium channel blockers reduced the risk of severe hypertension (risk ratio [RR], 0.70; 95% CI 0.56-0.88), there was no difference when methyldopa and calcium channel blockers were compared with β-blockers (RR, 1.18; 95% CI, 0.95- 1.48).43 Similarly, there were no differences in the risks of preeclampsia, fetal or neonatal death, small for gestational age, or preterm delivery of less than 37 weeks’ gestation when other antihypertensives were compared with calcium channel blockers or with β-blockers.45 Thus, the superiority of nifedipine compared with labetalol or vice versa is not established.
ACOG recommends low-dose aspirin for prophylaxis of preeclampsia in patients with CHTN during pregnancy. However, more recent studies have failed to find that aspirin prophylaxis in women with CHTN is beneficial in reducing the risk of preeclampsia.44 Although this recommendation is yet to change, clinical care providers should recognize the limitations of the efficacy of aspirin in patients with CHTN who already may have cardiovascular remodeling of their blood vessels and therefore may not benefit from aspirin prophylaxis.
There are active areas requiring investigation for patients with CHTN in pregnancy. The diagnosis of CHTN during pregnancy, superimposed preeclampsia, new-onset preeclampsia, and gestational hypertension continue to rest on the thresholds of 140/90 mm Hg during pregnancy despite multiple large observational studies showing increased risks of preeclampsia, small for gestational age, low birth weight, and preterm delivery in patients with persistent BPs of 130-139/80-89 mm Hg.5-8,45-48 Logistic concerns about increased health care utilization for up to 1 in 4 pregnant patients if the ACC/AHA criteria were applied to pregnancy—even though no effective proven prophylaxis or treatment exists—likely contributes to the hesitancy to adopt the ACC/AHA guidelines in pregnancy. Ongoing research to identify effective interventions may tip this balance.
Conclusion
The incidence of CHTN in pregnancy is rising and is associated with maternal and neonatal adverse outcomes. Pregnant patients should be treated with antihypertensive medications to less than 140/90 mm Hg. Optimal treatment BP goals, diagnostic BP threshold in pregnancy, and long-term cardiovascular outcomes following treatment started in pregnancy are active areas of research.
References
1. Cunningham FG, Leveno KJ, Bloom SL, eds. Chronic hypertension. In: Williams Obstetrics, 25e. McGraw-Hill Education; 2018.
2. American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins—Obstetrics. ACOG practice bulletin No. 203: chronic hypertension in pregnancy. Obstet Gynecol. 2019;133(1):e26-e50. doi:10.1097/AOG.0000000000003020
3. Sinkey RG, Oparil S. Lower blood pressure thresholds raise the bar in pregnancy. Circ Res. 2019;125(2):195-197. doi:10.1161/CIRCRESAHA.119.315384
4. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Hypertension. 2018;71(6):e13-e115. doi:10.1161/HYP.0000000000000065
5. Sutton EF, Hauspurg A, Caritis SN, Powers RW, Catov JM. Maternal outcomes associated with lower range stage 1 hypertension. Obstet Gynecol. 2018;132(4):843-849. doi:10.1097/AOG.0000000000002870
6. Greenberg VR, Silasi M, Lundsberg LS, et al. Perinatal outcomes in women with elevated blood pressure and stage 1 hypertension. Am J Obstet Gynecol. 2021;224(5):521.e1-521.e11. doi:10.1016/j.ajog.2020.10.049
7. Norton E, Shofer F, Schwartz H, Dugoff L. Adverse perinatal outcomes associated with stage 1 hypertension in pregnancy: a retrospective cohort study. Am J Perinatol. Published online November 2021. doi:10.1055/s-0041-1739470
8. Liu J, Tao L, Cao Y, et al. Stage 1 hypertension defined by the 2017 American College of Cardiology/American Heart Association guideline and risk of adverse birth outcomes in Eastern China. J Hypertens. 2020;38(6):1090-1102. doi:10.1097/HJH.0000000000002380
9. Topel ML, Duncan EM, Krishna I, Badell ML, Vaccarino V, Quyyumi AA. Estimated impact of the 2017 American College of Cardiology/American Heart Association blood pressure guidelines on reproductive-aged women. Hypertension. 2018;72(4):e39-e42. doi:10.1161/HYPERTENSIONAHA.118.11660
10. Bello NA, Zhou H, Cheetham TC, et al. Prevalence of hypertension among pregnant women when using the 2017 American College of Cardiology/American Heart Association blood pressure guidelines and association with maternal and fetal outcomes. JAMA Netw Open. 2021;4(3):e213808. doi:10.1001/JAMANETWORKOPEN.2021.3808
11. High blood pressure. American Heart Association. Accessed December 13, 2022. https://www.heart.org/en/health-topics/high-blood-pressure
12. ACOG committee opinion No. 767: emergent therapy for acute-onset, severe hypertension during pregnancy and the postpartum period. Obstet Gynecol. 2019;133(2):e174-e180. doi:10.1097/AOG.0000000000003075
13. Garovic VD, Dechend R, Easterling T, et al. Hypertension in pregnancy: diagnosis, blood pressure goals, and pharmacotherapy: a scientific statement from the American Heart Association. Hypertension. 2022;79(2):e21-e41. doi:10.1161/HYP.0000000000000208
14. How to accurately measure blood pressure at home. American Heart Association. May 22, 2020. Accessed March 16, 2023. https://www.heart.org/en/news/2020/05/22/how-to-accurately-measure-blood-pressure-at-home
15. Creanga AA, Catalano PM, Bateman BT. Obesity in pregnancy. N Engl J Med. 2022;387(3):248-259. doi:10.1056/NEJMRA1801040
16. Chen TK, Knicely DH, Grams ME. Chronic kidney disease diagnosis and management: a review. JAMA. 2019;322(13):1294-1304. doi:10.1001/JAMA.2019.14745
17. Di Palo KE, Barone NJ. Hypertension and heart failure: prevention, targets, and treatment. Heart Fail Clin. 2020;16(1):99-106. doi:10.1016/J.HFC.2019.09.001
18. Rakugi H, Yu H, Kamitani A, et al. Links between hypertension and myocardial infarction. Am Heart J. 1996;132(1 Pt 2 Su):213-221.
19. Pedrinelli R, Ballo P, Fiorentini C, et al. Hypertension and acute myocardial infarction: an overview. J Cardiovasc Med (Hagerstown). 2012;13(3):194-202. doi:10.2459/JCM.0B013E3283511EE2
20. Wajngarten M, Silva GS. Hypertension and stroke: update on treatment. Eur Cardiol. 2019;14(2):111-115. doi:10.15420/ECR.2019.11.1
21. Petersen EE, Davis NL, Goodman D, et al. Vital signs: pregnancy-related deaths, United States, 2011-2015, and strategies for prevention, 13 states, 2013-2017. MMWR Morb Mortal Wkly Rep. 2019;68(18):423-429. doi:10.15585/MMWR.MM6818E1
22. Ely DM, Driscoll AK, Matthews TJ. Infant mortality by age at death in the United States, 2016. NCHS Data Brief. 2018;(326):1-8.
23. Vanek M, Sheiner E, Levy A, Mazor M. Chronic hypertension and the risk for adverse pregnancy outcome after superimposed pre-eclampsia. Int J Gynaecol Obstet. 2004;86(1):7-11. doi:10.1016/j.ijgo.2004.03.006
24. Oben A, Szychowski JM, Ketch P, et al. Progression to severe chronic hypertension 5-7 years after a pregnancy with mild chronic hypertension. Obstet Gynecol. 2022;140(4):546-553. doi:10.1097/AOG.0000000000004925
25. Timpka S, Stuart JJ, Tanz LJ, Rimm EB, Franks PW, Rich-Edwards JW. Lifestyle in progression from hypertensive disorders of pregnancy to chronic hypertension in Nurses’ Health Study II: observational cohort study. BMJ. 2017;358:j3024. doi:10.1136/BMJ.J3024
26. Hall ME, Cohen JB, Ard JD, et al. Weight-loss strategies for prevention and treatment of hypertension: a scientific statement from the American Heart Association. Hypertension. 2021;78(5):e38-e50. doi:10.1161/HYP.0000000000000202
27. Fuchs FD, Chambless LE. Is the cardioprotective effect of alcohol real? Alcohol. 2007;41(6):399-402. doi:10.1016/J.ALCOHOL.2007.05.004
28. Börjesson M, Onerup A, Lundqvist S, Dahlöf B. Physical activity and exercise lower blood pressure in individuals with hypertension: narrative review of 27 RCTs. Br J Sports Med. 2016;50(6):356-361. doi:10.1136/BJSPORTS-2015-095786
29. Arterburn DE, Telem DA, Kushner RF, Courcoulas AP. Benefits and risks of bariatric surgery in adults: a review. JAMA. 2020;324(9):879-887. doi:10.1001/JAMA.2020.12567
30. Bundy JD, Li C, Stuchlik P, et al. Systolic blood pressure reduction and risk of cardiovascular disease and mortality: a systematic review and network meta-analysis. JAMA Cardiol. 2017;2(7):775-781. doi:10.1001/JAMACARDIO.2017.1421
31. Ettehad D, Emdin CA, Kiran A, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet. 2016;387(10022):957-967. doi:10.1016/S0140-6736(15)01225-8
32. Clinical guidance for the integration of the findings of the chronic hypertension and pregnancy (CHAP) study. American College of Obstetricians and Gynecologists. April 2022. Accessed March 16, 2023. https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2022/04/clinical-guidance-for-the-integration-of-the-findings-of-the-chronic-hypertension-and-pregnancy-chap-study
34. Tita AT, Szychowski JM, Boggess K, et al. Treatment for mild chronic hypertension during pregnancy. N Engl J Med. 2022;386(19):1781-1792. doi:10.1056/NEJMoa2201295
34. Bailey E, Hoppe KK. Treatment for mild chronic hypertension during pregnancy: is tighter better? Am J Obstet Gynecol. 2023;228(1):S2. doi:10.1016/j.ajog.2022.11.004
35. Wright JT Jr, Williamson JD, Whelton PK, et al; SPRINT Research Group. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373(22):2103-2116. doi:10.1056/NEJMoa1511939
36. Carey RM, Moran AE, Whelton PK. Treatment of hypertension: a review. JAMA. 2022;328(18):1849-1861. doi:10.1001/JAMA.2022.19590
37. Fu J, Tomlinson G, Feig DS. Increased risk of major congenital malformations in early pregnancy use of angiotensin-converting-enzyme inhibitors and angiotensin-receptor-blockers: a meta-analysis. Diabetes Metab Res Rev. 2021;37(8):e3453. doi:10.1002/DMRR.3453
38. Ferrer RL, Sibai BM, Mulrow CD, Chiquette E, Stevens KR, Cornell J. Management of mild chronic hypertension during pregnancy: a review. Obstet Gynecol. 2000;96(5 Pt 2):849-860. doi:10.1016/S0029-7844(00)00938-8
39. SMFM statement: antihypertensive therapy for mild chronic hypertension in pregnancy: the CHAP trial. Society for Maternal-Fetal Medicine. April 19, 2022. Accessed December 13, 2022. https://www.smfm.org/publications/439-smfm-statement-antihypertensive-therapy-for-mild-chronic-hypertension-in-pregnancy-the-chap-trial
40. Magee LA, von Dadelszen P, Rey E, et al. Less-tight versus tight control of hypertension in pregnancy. N Engl J Med. 2015;372(5):407-417. doi:10.1056/NEJMoa1404595
41. Hauspurg A, Sutton EF, Catov JM, Caritis SN. Aspirin effect on adverse pregnancy outcomes associated with stage 1 hypertension in a high-risk cohort. Hypertension. 2018;72(1):202-207. doi:10.1161/HYPERTENSIONAHA.118.11196
42. Huai J, Lin L, Juan J, et al. Preventive effect of aspirin on preeclampsia in high-risk pregnant women with stage 1 hypertension. J Clin Hypertens (Greenwich). 2021;23(5):1060-1067. doi:10.1111/jch.14149
43. Abalos E, Duley L, Steyn DW, Gialdini C. Antihypertensive drug therapy for mild to moderate hypertension during pregnancy. Cochrane Database Syst Rev. 2018;10(10):CD002252. doi:10.1002/14651858.CD002252.pub4
44. Rolnik DL, Nicolaides KH, Poon LC. Prevention of preeclampsia with aspirin. Am J Obstet Gynecol. 2022;226(2S):S1108-S1119. doi:10.1016/j.ajog.2020.08.045
45. Sabol BA, Porcelli B, Diveley E, et al. Defining the risk profile of women with stage 1 hypertension: a time to event analysis. Am J Obstet Gynecol MFM. 2021;3(4):100376. doi:10.1016/j.ajogmf.2021.100376
46. Li Q, Zheng L, Gu Y, et al. Early pregnancy stage 1 hypertension and high mean arterial pressure increased risk of adverse pregnancy outcomes in Shanghai, China. J Hum Hypertens. Published online June 1, 2021. doi:10.1038/s41371-021-00523-6
47. Wu DD, Gao L, Huang O, et al. Increased adverse pregnancy outcomes associated with stage 1 hypertension in a low-risk cohort: evidence from 47 874 cases. Hypertension. 2020;75(3):772-780. doi:10.1161/HYPERTENSIONAHA.119.14252
48. Bateman BT, Bansil P, Hernandez-Diaz S, Mhyre JM, Callaghan WM, Kuklina EV. Prevalence, trends, and outcomes of chronic hypertension: a nationwide sample of delivery admissions. Am J Obstet Gynecol. 2012;206(2):134.e1-134.e1348. doi:10.1016/J.AJOG.2011.10.878