Infection
Endothelial AHR activity prevents lung barrier disruption in viral infection
Abstract
Disruption of the lung endothelial–epithelial cell barrier following respiratory virus infection causes cell and fluid accumulation in the air spaces and compromises vital gas exchange function1. Endothelial dysfunction can exacerbate tissue damage2,3, yet it is unclear whether the lung endothelium promotes host resistance against viral pathogens. Here we show that the environmental sensor aryl hydrocarbon receptor (AHR) is highly active in lung endothelial cells and protects against influenza-induced lung vascular leakage. Loss of AHR in endothelia exacerbates lung damage and promotes the infiltration of red blood cells and leukocytes into alveolar air spaces. Moreover, barrier protection is compromised and host susceptibility to secondary bacterial infections is increased when endothelial AHR is missing. AHR engages tissue-protective transcriptional networks in endothelia, including the vasoactive apelin–APJ peptide system4, to prevent a dysplastic and apoptotic response in airway epithelial cells. Finally, we show that protective AHR signalling in lung endothelial cells is dampened by the infection itself. Maintenance of protective AHR function requires a diet enriched in naturally occurring AHR ligands, which activate disease tolerance pathways in lung endothelia to prevent tissue damage. Our findings demonstrate the importance of endothelial function in lung barrier immunity. We identify a gut–lung axis that affects lung damage following encounters with viral pathogens, linking dietary composition and intake to host fitness and inter-individual variations in disease outcome.
This is a preview of subscription content, access via your institution
Access options
style{display:none!important}.LiveAreaSection-193358632 *{align-content:stretch;align-items:stretch;align-self:auto;animation-delay:0s;animation-direction:normal;animation-duration:0s;animation-fill-mode:none;animation-iteration-count:1;animation-name:none;animation-play-state:running;animation-timing-function:ease;azimuth:center;backface-visibility:visible;background-attachment:scroll;background-blend-mode:normal;background-clip:borderBox;background-color:transparent;background-image:none;background-origin:paddingBox;background-position:0 0;background-repeat:repeat;background-size:auto auto;block-size:auto;border-block-end-color:currentcolor;border-block-end-style:none;border-block-end-width:medium;border-block-start-color:currentcolor;border-block-start-style:none;border-block-start-width:medium;border-bottom-color:currentcolor;border-bottom-left-radius:0;border-bottom-right-radius:0;border-bottom-style:none;border-bottom-width:medium;border-collapse:separate;border-image-outset:0s;border-image-repeat:stretch;border-image-slice:100%;border-image-source:none;border-image-width:1;border-inline-end-color:currentcolor;border-inline-end-style:none;border-inline-end-width:medium;border-inline-start-color:currentcolor;border-inline-start-style:none;border-inline-start-width:medium;border-left-color:currentcolor;border-left-style:none;border-left-width:medium;border-right-color:currentcolor;border-right-style:none;border-right-width:medium;border-spacing:0;border-top-color:currentcolor;border-top-left-radius:0;border-top-right-radius:0;border-top-style:none;border-top-width:medium;bottom:auto;box-decoration-break:slice;box-shadow:none;box-sizing:border-box;break-after:auto;break-before:auto;break-inside:auto;caption-side:top;caret-color:auto;clear:none;clip:auto;clip-path:none;color:initial;column-count:auto;column-fill:balance;column-gap:normal;column-rule-color:currentcolor;column-rule-style:none;column-rule-width:medium;column-span:none;column-width:auto;content:normal;counter-increment:none;counter-reset:none;cursor:auto;display:inline;empty-cells:show;filter:none;flex-basis:auto;flex-direction:row;flex-grow:0;flex-shrink:1;flex-wrap:nowrap;float:none;font-family:initial;font-feature-settings:normal;font-kerning:auto;font-language-override:normal;font-size:medium;font-size-adjust:none;font-stretch:normal;font-style:normal;font-synthesis:weight style;font-variant:normal;font-variant-alternates:normal;font-variant-caps:normal;font-variant-east-asian:normal;font-variant-ligatures:normal;font-variant-numeric:normal;font-variant-position:normal;font-weight:400;grid-auto-columns:auto;grid-auto-flow:row;grid-auto-rows:auto;grid-column-end:auto;grid-column-gap:0;grid-column-start:auto;grid-row-end:auto;grid-row-gap:0;grid-row-start:auto;grid-template-areas:none;grid-template-columns:none;grid-template-rows:none;height:auto;hyphens:manual;image-orientation:0deg;image-rendering:auto;image-resolution:1dppx;ime-mode:auto;inline-size:auto;isolation:auto;justify-content:flexStart;left:auto;letter-spacing:normal;line-break:auto;line-height:normal;list-style-image:none;list-style-position:outside;list-style-type:disc;margin-block-end:0;margin-block-start:0;margin-bottom:0;margin-inline-end:0;margin-inline-start:0;margin-left:0;margin-right:0;margin-top:0;mask-clip:borderBox;mask-composite:add;mask-image:none;mask-mode:matchSource;mask-origin:borderBox;mask-position:0 0;mask-repeat:repeat;mask-size:auto;mask-type:luminance;max-height:none;max-width:none;min-block-size:0;min-height:0;min-inline-size:0;min-width:0;mix-blend-mode:normal;object-fit:fill;object-position:50% 50%;offset-block-end:auto;offset-block-start:auto;offset-inline-end:auto;offset-inline-start:auto;opacity:1;order:0;orphans:2;outline-color:initial;outline-offset:0;outline-style:none;outline-width:medium;overflow:visible;overflow-wrap:normal;overflow-x:visible;overflow-y:visible;padding-block-end:0;padding-block-start:0;padding-bottom:0;padding-inline-end:0;padding-inline-start:0;padding-left:0;padding-right:0;padding-top:0;page-break-after:auto;page-break-before:auto;page-break-inside:auto;perspective:none;perspective-origin:50% 50%;pointer-events:auto;position:static;quotes:initial;resize:none;right:auto;ruby-align:spaceAround;ruby-merge:separate;ruby-position:over;scroll-behavior:auto;scroll-snap-coordinate:none;scroll-snap-destination:0 0;scroll-snap-points-x:none;scroll-snap-points-y:none;scroll-snap-type:none;shape-image-threshold:0;shape-margin:0;shape-outside:none;tab-size:8;table-layout:auto;text-align:initial;text-align-last:auto;text-combine-upright:none;text-decoration-color:currentcolor;text-decoration-line:none;text-decoration-style:solid;text-emphasis-color:currentcolor;text-emphasis-position:over right;text-emphasis-style:none;text-indent:0;text-justify:auto;text-orientation:mixed;text-overflow:clip;text-rendering:auto;text-shadow:none;text-transform:none;text-underline-position:auto;top:auto;touch-action:auto;transform:none;transform-box:borderBox;transform-origin:50% 50%0;transform-style:flat;transition-delay:0s;transition-duration:0s;transition-property:all;transition-timing-function:ease;vertical-align:baseline;visibility:visible;white-space:normal;widows:2;width:auto;will-change:auto;word-break:normal;word-spacing:normal;word-wrap:normal;writing-mode:horizontalTb;z-index:auto;-webkit-appearance:none;-moz-appearance:none;-ms-appearance:none;appearance:none;margin:0}.LiveAreaSection-193358632{width:100%}.LiveAreaSection-193358632 .login-option-buybox{display:block;width:100%;font-size:17px;line-height:30px;color:#222;padding-top:30px;font-family:Harding,Palatino,serif}.LiveAreaSection-193358632 .additional-access-options{display:block;font-weight:700;font-size:17px;line-height:30px;color:#222;font-family:Harding,Palatino,serif}.LiveAreaSection-193358632 .additional-login>li:not(:first-child)::before{transform:translateY(-50%);content:””;height:1rem;position:absolute;top:50%;left:0;border-left:2px solid #999}.LiveAreaSection-193358632 .additional-login>li:not(:first-child){padding-left:10px}.LiveAreaSection-193358632 .additional-login>li{display:inline-block;position:relative;vertical-align:middle;padding-right:10px}.BuyBoxSection-683559780{display:flex;flex-wrap:wrap;flex:1;flex-direction:row-reverse;margin:-30px -15px 0}.BuyBoxSection-683559780 .box-inner{width:100%;height:100%}.BuyBoxSection-683559780 .readcube-buybox{background-color:#f3f3f3;flex-shrink:1;flex-grow:1;flex-basis:255px;background-clip:content-box;padding:0 15px;margin-top:30px}.BuyBoxSection-683559780 .subscribe-buybox{background-color:#f3f3f3;flex-shrink:1;flex-grow:4;flex-basis:300px;background-clip:content-box;padding:0 15px;margin-top:30px}.BuyBoxSection-683559780 .subscribe-buybox-nature-plus{background-color:#f3f3f3;flex-shrink:1;flex-grow:4;flex-basis:100%;background-clip:content-box;padding:0 15px;margin-top:30px}.BuyBoxSection-683559780 .title-readcube,.BuyBoxSection-683559780 .title-buybox{display:block;margin:0;margin-right:10%;margin-left:10%;font-size:24px;line-height:32px;color:#222;padding-top:30px;text-align:center;font-family:Harding,Palatino,serif}.BuyBoxSection-683559780 .title-asia-buybox{display:block;margin:0;margin-right:5%;margin-left:5%;font-size:24px;line-height:32px;color:#222;padding-top:30px;text-align:center;font-family:Harding,Palatino,serif}.BuyBoxSection-683559780 .asia-link{color:#069;cursor:pointer;text-decoration:none;font-size:1.05em;font-family:-apple-system,BlinkMacSystemFont,”Segoe UI”,Roboto,Oxygen-Sans,Ubuntu,Cantarell,”Helvetica Neue”,sans-serif;line-height:1.05em6}.BuyBoxSection-683559780 .access-readcube{display:block;margin:0;margin-right:10%;margin-left:10%;font-size:14px;color:#222;padding-top:10px;text-align:center;font-family:-apple-system,BlinkMacSystemFont,”Segoe UI”,Roboto,Oxygen-Sans,Ubuntu,Cantarell,”Helvetica Neue”,sans-serif;line-height:20px}.BuyBoxSection-683559780 .access-asia-buybox{display:block;margin:0;margin-right:5%;margin-left:5%;font-size:14px;color:#222;padding-top:10px;text-align:center;font-family:-apple-system,BlinkMacSystemFont,”Segoe UI”,Roboto,Oxygen-Sans,Ubuntu,Cantarell,”Helvetica Neue”,sans-serif;line-height:20px}.BuyBoxSection-683559780 .access-buybox{display:block;margin:0;margin-right:10%;margin-left:10%;font-size:14px;color:#222;opacity:.8px;padding-top:10px;text-align:center;font-family:-apple-system,BlinkMacSystemFont,”Segoe UI”,Roboto,Oxygen-Sans,Ubuntu,Cantarell,”Helvetica Neue”,sans-serif;line-height:20px}.BuyBoxSection-683559780 .price-buybox{display:block;font-size:30px;color:#222;font-family:-apple-system,BlinkMacSystemFont,”Segoe UI”,Roboto,Oxygen-Sans,Ubuntu,Cantarell,”Helvetica Neue”,sans-serif;padding-top:30px;text-align:center}.BuyBoxSection-683559780 .price-buybox-to{display:block;font-size:30px;color:#222;font-family:-apple-system,BlinkMacSystemFont,”Segoe UI”,Roboto,Oxygen-Sans,Ubuntu,Cantarell,”Helvetica Neue”,sans-serif;text-align:center}.BuyBoxSection-683559780 .price-info-text{font-size:16px;padding-right:10px;color:#222;font-family:-apple-system,BlinkMacSystemFont,”Segoe UI”,Roboto,Oxygen-Sans,Ubuntu,Cantarell,”Helvetica Neue”,sans-serif}.BuyBoxSection-683559780 .price-value{font-size:30px;font-family:-apple-system,BlinkMacSystemFont,”Segoe UI”,Roboto,Oxygen-Sans,Ubuntu,Cantarell,”Helvetica Neue”,sans-serif}.BuyBoxSection-683559780 .price-per-period{font-family:-apple-system,BlinkMacSystemFont,”Segoe UI”,Roboto,Oxygen-Sans,Ubuntu,Cantarell,”Helvetica Neue”,sans-serif}.BuyBoxSection-683559780 .price-from{font-size:14px;padding-right:10px;color:#222;font-family:-apple-system,BlinkMacSystemFont,”Segoe UI”,Roboto,Oxygen-Sans,Ubuntu,Cantarell,”Helvetica Neue”,sans-serif;line-height:20px}.BuyBoxSection-683559780 .issue-buybox{display:block;font-size:13px;text-align:center;color:#222;font-family:-apple-system,BlinkMacSystemFont,”Segoe UI”,Roboto,Oxygen-Sans,Ubuntu,Cantarell,”Helvetica Neue”,sans-serif;line-height:19px}.BuyBoxSection-683559780 .no-price-buybox{display:block;font-size:13px;line-height:18px;text-align:center;padding-right:10%;padding-left:10%;padding-bottom:20px;padding-top:30px;color:#222;font-family:-apple-system,BlinkMacSystemFont,”Segoe UI”,Roboto,Oxygen-Sans,Ubuntu,Cantarell,”Helvetica Neue”,sans-serif}.BuyBoxSection-683559780 .vat-buybox{display:block;margin-top:5px;margin-right:20%;margin-left:20%;font-size:11px;color:#222;padding-top:10px;padding-bottom:15px;text-align:center;font-family:-apple-system,BlinkMacSystemFont,”Segoe UI”,Roboto,Oxygen-Sans,Ubuntu,Cantarell,”Helvetica Neue”,sans-serif;line-height:17px}.BuyBoxSection-683559780 .tax-buybox{display:block;width:100%;color:#222;padding:20px 16px;text-align:center;font-family:-apple-system,BlinkMacSystemFont,”Segoe UI”,Roboto,Oxygen-Sans,Ubuntu,Cantarell,”Helvetica Neue”,sans-serif;line-height:NaNpx}.BuyBoxSection-683559780 .button-container{display:flex;padding-right:20px;padding-left:20px;justify-content:center}.BuyBoxSection-683559780 .button-container>*{flex:1px}.BuyBoxSection-683559780 .button-container>a:hover,.Button-505204839:hover,.Button-1078489254:hover,.Button-2496381730:hover{text-decoration:none}.BuyBoxSection-683559780 .readcube-button{background:#fff;margin-top:30px}.BuyBoxSection-683559780 .button-asia{background:#069;border:1px solid #069;border-radius:0;cursor:pointer;display:block;padding:9px;outline:0;text-align:center;text-decoration:none;min-width:80px;margin-top:75px}.BuyBoxSection-683559780 .button-label-asia,.ButtonLabel-3869432492,.ButtonLabel-3296148077,.ButtonLabel-1651148777{display:block;color:#fff;font-size:17px;line-height:20px;font-family:-apple-system,BlinkMacSystemFont,”Segoe UI”,Roboto,Oxygen-Sans,Ubuntu,Cantarell,”Helvetica Neue”,sans-serif;text-align:center;text-decoration:none;cursor:pointer}.Button-505204839,.Button-1078489254,.Button-2496381730{background:#069;border:1px solid #069;border-radius:0;cursor:pointer;display:block;padding:9px;outline:0;text-align:center;text-decoration:none;min-width:80px;max-width:320px;margin-top:10px}.Button-505204839 .readcube-label,.Button-1078489254 .readcube-label,.Button-2496381730 .readcube-label{color:#069}
/* style specs end */
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Rent or buy this article
Prices vary by article type
from$1.95
to$39.95
Prices may be subject to local taxes which are calculated during checkout



Data availability
Sequencing data are available in the Gene Expression Omnibus under accession codes GSE203427 and GSE225958. Source data are provided with this paper.
References
-
Matthay, M. A et al. Acute respiratory distress syndrome. Nat. Rev. Dis. Primers 5, 18 (2019).
Google Scholar
-
Bonaventura, A. et al. Endothelial dysfunction and immunothrombosis as key pathogenic mechanisms in COVID-19. Nat. Rev. Immunol. 21, 319–329 (2021).
Google Scholar
-
Teijaro, J. R. et al. Endothelial cells are central orchestrators of cytokine amplification during influenza virus infection. Cell 146, 980–991 (2011).
Google Scholar
-
Kleinz, M. J., Skepper, J. N. & Davenport, A. P. Immunocytochemical localisation of the apelin receptor, APJ, to human cardiomyocytes, vascular smooth muscle and endothelial cells. Regul. Pept. 126, 233–240 (2005).
Google Scholar
-
Krausgruber, T. et al. Structural cells are key regulators of organ-specific immune responses. Nature 583, 296–302 (2020).
Google Scholar
-
Hogan, B. L. M. et al. Repair and regeneration of the respiratory system: complexity, plasticity, and mechanisms of lung stem cell function. Cell Stem Cell 15, 123–138 (2014).
Google Scholar
-
Fernanda de Mello Costa, M., Weiner, A. I. & Vaughan, A. E. Basal-like progenitor cells: a review of dysplastic alveolar regeneration and remodeling in lung repair. Stem Cell Rep. 15, 1015–1025 (2020).
Google Scholar
-
Basil, M. C. et al. The cellular and physiological basis for lung repair and regeneration: past, present, and future. Cell Stem Cell 26, 482–502 (2020).
Google Scholar
-
Zhao, G. et al. Regeneration of the pulmonary vascular endothelium after viral pneumonia requires COUP-TF2. Sci. Adv. 6, eabc4493 (2020).
Google Scholar
-
Niethamer, T. K. et al. Defining the role of pulmonary endothelial cell heterogeneity in the response to acute lung injury. eLife 9, e53072 (2020).
Google Scholar
-
Ding, B. Sen et al. Endothelial-derived angiocrine signals induce and sustain regenerative lung alveolarization. Cell 147, 539–553 (2011).
Google Scholar
-
Lee, J. H. et al. Lung stem cell differentiation in mice directed by endothelial cells via a BMP4–NFATc1–thrombospondin-1 axis. Cell 156, 440–455 (2014).
Google Scholar
-
Rafii, S. et al. Platelet-derived SDF-1 primes the pulmonary capillary vascular niche to drive lung alveolar regeneration. Nat. Cell Biol. 17, 123–136 (2015).
Google Scholar
-
Stockinger, B., Di Meglio, P., Gialitakis, M. & Duarte, J. H. The aryl hydrocarbon receptor: multitasking in the immune system. Annu. Rev. Immunol. 32, 403–432 (2014).
Google Scholar
-
Denison, M. S. & Nagy, S. R. Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Annu. Rev. Pharmacol. Toxicol. 43, 309–334 (2003).
Google Scholar
-
Wincent, E. et al. Inhibition of cytochrome P4501-dependent clearance of the endogenous agonist FICZ as a mechanism for activation of the aryl hydrocarbon receptor. Proc. Natl Acad. Sci. USA 109, 4479–4484 (2012).
Google Scholar
-
Chiaro, C. R., Patel, R. D., Marcus, C. B. & Perdew, G. H. Evidence for an aryl hydrocarbon receptor-mediated cytochrome P450 autoregulatory pathway. Mol. Pharmacol. 72, 1369–1379 (2007).
Google Scholar
-
Li, Y. et al. Exogenous stimuli maintain intraepithelial lymphocytes via aryl hydrocarbon receptor activation. Cell 147, 629–640 (2011).
Google Scholar
-
Kiss, E. A. et al. Natural aryl hydrocarbon receptor ligands control organogenesis of intestinal lymphoid follicles. Science 334, 1561–1565 (2011).
Google Scholar
-
Qiu, J. et al. The aryl hydrocarbon receptor regulates gut immunity through modulation of innate lymphoid cells. Immunity 36, 92–104 (2012).
Google Scholar
-
Schiering, C. et al. Feedback control of AHR signalling regulates intestinal immunity. Nature 542, 242–245 (2017).
Google Scholar
-
Metidji, A. et al. The environmental sensor AHR protects from inflammatory damage by maintaining intestinal stem cell homeostasis and barrier integrity. Immunity https://doi.org/10.1016/j.immuni.2018.07.010 (2018).
-
Stockinger, B., Shah, K. & Wincent, E. AHR in the intestinal microenvironment: safeguarding barrier function. Nat. Rev. Gastroenterol. Hepatol. 18, 559–570 (2021).
Google Scholar
-
Gronke, K. et al. Interleukin-22 protects intestinal stem cells against genotoxic stress. Nature https://doi.org/10.1038/s41586-019-0899-7 (2019).
-
Yamada, T. et al. Constitutive aryl hydrocarbon receptor signaling constrains type I interferon-mediated antiviral innate defense. Nat. Immunol. 17, 687–694 (2016).
Google Scholar
-
Villa, M. et al. The aryl hydrocarbon receptor controls cyclin O to promote epithelial multiciliogenesis. Nat. Commun. 7, 12652 (2016).
Google Scholar
-
Moura-Alves, P. et al. AhR sensing of bacterial pigments regulates antibacterial defence. Nature 512, 387–392 (2014).
Google Scholar
-
Diny, N. L. et al. The aryl hydrocarbon receptor contributes to tissue adaptation of intestinal eosinophils in mice. J. Exp. Med. 219, e20210970 (2022).
Google Scholar
-
Henderson, C. J. et al. Application of a novel regulatable Cre recombinase system to define the role of liver and gut metabolism in drug oral bioavailability. Biochem. J. 465, 479–488 (2015).
Google Scholar
-
Schupp, J. C. et al. Integrated single-cell atlas of endothelial cells of the human lung. Circulation 144, 286–302 (2021).
Google Scholar
-
Dragin, N. et al. Phenotype of the Cyp1a1/1a2/1b1(–/–) triple-knockout mouse. Mol. Pharmacol. 73, 1844–1856 (2008).
Google Scholar
-
Medzhitov, R., Schneider, D. S. & Soares, M. P. Disease tolerance as a defense strategy. Science 335, 936–942 (2012).
Google Scholar
-
Shimada, T. et al. Arylhydrocarbon receptor-dependent induction of liver and lung cytochromes P450 1A1, 1A2, and 1B1 by polycyclic aromatic hydrocarbons and polychlorinated biphenyls in genetically engineered C57BL/6J mice. Carcinogenesis 23, 1199–1207 (2002).
Google Scholar
-
Kumar, P. A. et al. Distal airway stem cells yield alveoli in vitro and during lung regeneration following H1N1 influenza infection. Cell 147, 525–538 (2011).
Google Scholar
-
Vaughan, A. E. et al. Lineage-negative progenitors mobilize to regenerate lung epithelium after major injury. Nature 517, 621–625 (2015).
Google Scholar
-
Zuo, W. et al. p63+Krt5+ distal airway stem cells are essential for lung regeneration. Nature 517, 616–620 (2014).
Google Scholar
-
Quantius, J. et al. Influenza virus infects epithelial stem/progenitor cells of the distal lung: impact on Fgfr2b-driven epithelial repair. PLoS Pathog. 12, e1005544 (2016).
Google Scholar
-
McQualter, J. L., Yuen, K., Williams, B. & Bertoncello, I. Evidence of an epithelial stem/progenitor cell hierarchy in the adult mouse lung. Proc. Natl Acad. Sci. USA 107, 1414–1419 (2010).
Google Scholar
-
Cox, C. M., D’Agostino, S. L., Miller, M. K., Heimark, R. L. & Krieg, P. A. Apelin, the ligand for the endothelial G-protein-coupled receptor, APJ, is a potent angiogenic factor required for normal vascular development of the frog embryo. Dev. Biol. 296, 177–189 (2006).
Google Scholar
-
Gillich, A. et al. Capillary cell-type specialization in the alveolus. Nature 586, 785–789 (2020).
Google Scholar
-
He, Q. et al. Apelin-36 protects against lipopolysaccharide-induced acute lung injury by inhibiting the ASK1/MAPK signaling pathway. Mol. Med. Rep. 23, 6 (2021).
-
Kong, X. et al. Apelin-13-Mediated AMPK ameliorates endothelial barrier dysfunction in acute lung injury mice via improvement of mitochondrial function and autophagy. Int. Immunopharmacol. 101, 108230 (2021).
-
Fan, X. F. et al. The apelin–APJ axis is an endogenous counterinjury mechanism in experimental acute lung injury. Chest 147, 969–978 (2015).
Google Scholar
-
Macaluso, N. J. M., Pitkin, S. L., Maguire, J. J., Davenport, A. P. & Glen, R. C. Discovery of a competitive apelin receptor (APJ) antagonist. ChemMedChem 6, 1017–1023 (2011).
Google Scholar
-
Bjeldanes, L. F., Kim, J. Y., Grose, K. R., Bartholomew, J. C. & Bradfield, C. A. Aromatic hydrocarbon responsiveness-receptor agonists generated from indole-3-carbinol in vitro and in vivo: comparisons with 2,3,7,8-tetrachlorodibenzo-p-dioxin. Proc. Natl Acad. Sci. USA 88, 9543–9547 (1991).
Google Scholar
-
Wiggins, B. G. et al. Endothelial sensing of AHR ligands regulates intestinal homeostasis. Nature https://doi.org/10.1038/s41586-023-06508-4 (2023).
-
Degner, S. C., Papoutsis, A. J., Selmin, O. & Romagnolo, D. F. Targeting of aryl hydrocarbon receptor-mediated activation of cyclooxygenase-2 expression by the indole-3-carbinol metabolite 3,3′-diindolylmethane in breast cancer cells. J. Nutr. 139, 26–32 (2009).
Google Scholar
-
Rothhammer, V. & Quintana, F. J. The aryl hydrocarbon receptor: an environmental sensor integrating immune responses in health and disease. Nat. Rev. Immunol. 19, 184–197 (2019).
Google Scholar
-
Lebwohl, M. G. et al. Phase 3 trials of tapinarof cream for plaque psoriasis. N. Engl. J. Med. 385, 2219–2229 (2021).
Google Scholar
-
Zhu, Z. et al. Aryl hydrocarbon receptor in cutaneous vascular endothelial cells restricts psoriasis development by negatively regulating neutrophil recruitment. J. Invest. Dermatol. 140, 1233–1243.e9 (2020).
Google Scholar
-
Anderton, M. J. et al. Pharmacokinetics and tissue disposition of indole-3-carbinol and its acid condensation products after oral administration to mice. Clin. Cancer Res. 10, 5233–5241 (2004).
Google Scholar
-
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Google Scholar
-
Parrish, N., Hormozdiari, F. & Eskin, E. in Bioinformatics. The Impact of Accurate Quantification on Proteomic and Genetic Analysis and Research (ed. Liu, Y.) Ch. 2, 21–40 (Apple Academic Press, 2014).
-
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Google Scholar
-
The R Core Team. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2018).
-
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl Acad. Sci. USA 102, 15545–15550 (2005).
Google Scholar
-
Krämer, A., Green, J., Pollard, J. & Tugendreich, S. Causal analysis approaches in ingenuity pathway analysis. Bioinformatics 30, 523–530 (2014).
Google Scholar
Acknowledgements
We thank staff at the Crick flow cytometry, advanced sequencing, bioinformatics and animal facilities for excellent support; and J. Kohl for help and advice on RNAscope data generation and interpretation. This work was funded by the Francis Crick Institute, which receives its core funding from Cancer Research UK (FC002085), the UK Medical Research Council (FC002085) and the Wellcome Trust (FC002085).
Author information
Authors and Affiliations
Contributions
J.M. and A.W. conceived the idea and designed the experimental strategy. J.M., S.C., K.F., B.F., R.D., M.G. and L.M. designed and performed experiments and analysed data. P.C. performed the bioinformatics analysis. K.S. and B.S. provided key intellectual input and experimental tools. A.S.-B. and S.P. performed histopathological analyses and scoring. J.M. and A.W. wrote the manuscript. All authors edited the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks the anonymous reviewers for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Gating strategies for lung cell populations.
a–c, Gating strategy for lung endothelial cell and epithelial cell populations (a), RBCs and immune cells in the BALF day 6 post influenza virus infection (b), and lung lymphoid immune cell populations (c) analysed by flow cytometry.
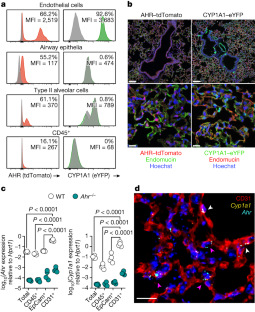
Extended Data Fig. 2 The AHR landscape in mouse and human lung endothelia.
a, Immunofluorescence staining of steady-state AHR-tdTomato and Cyp1a1-eYFP lung sections stained with the vascular endothelial marker endomucin or lymphatic marker LYVE-1, and Hoechst (blue). Scale bars, 100 μm (left panels), 20 μm (middle panels), 5 μm (right panels). Data are representative of three independent experiments with similar results. b, Representative histogram plots of AHR-tdTomato and Cyp1a1-eYFP expression in steady-state lung endothelial cells (CD31+PDPN–) and lymphatic endothelia (CD31+PDPN+) relative to B6 WT controls (grey). Mean fluorescence intensity (MFI). c, Frequency of lymphatic and vascular endothelial cells measured by flow cytometry. d, RNA-FISH analysis of WT steady-state lung. RNA probes for Ahr (cyan) and Cyp1a1 (yellow) and stained with E-Cadherin for epithelia. White arrowheads indicate Ahr expression in airway epithelial cells. Scale bar, 20 μm. Data are representative of four independent experiments with similar results. e, f, Expression of indicated genes in uniform manifold approximation and projection (UMAP) plots of mouse (e) and human (f) lung scRNA-seq datasets obtained from lungendothelialcellatlas.com. g, Primary human lung microvasculature endothelial cell (HMVEC-L) cultures were treated with AHR agonist FICZ or antagonist CH-223191 for 24 h and indicated gene expression was determined by qPCR (n = 6 biological replicates). Statistical analysis was performed using one-way ANOVA with Tukey’s post-test. Data are shown as mean±SEM. Data are shown as mean±SEM. ns, not significant.
Source data
Extended Data Fig. 3 Dampened pulmonary inflammation in CYP1-deficient mice.
a, b, Lung immune cell numbers were determined in the BALF on day 6 post infection (a) or in whole lung on indicated days post infection (b) in WT (n = 4) and Cyp1−/− (n = 5) mice by flow cytometry. c, d, BALF cytokine concentration in influenza virus infected WT and Cyp1−/− mice was determined on indicated days for IFN (n = 3) (c) or day 6 for remaining cytokines (d) post infection (n = 5). e, Histopathological analysis of WT (n = 4) and Cyp1−/− (n = 3) H&E lung sections on day 6 post infection. Black arrowheads indicate areas of perivascular, peribronchiolar, and intra-alveolar inflammatory cell infiltration. Scale bars, 500 μm (upper panels) and 100 μm (lower panels). All Data are representative of three to four independent experiments. Statistical analysis was performed using unpaired two-tailed Student’s t test (a, d), two-way ANOVA with Sidak’s post-test (b, c), or two-tailed Mann–Whitney U test (e) and significant P values are indicated on the graphs. Data are shown as mean±SEM. ns, not significant.
Source data
Extended Data Fig. 4 CYP1 deficiency confers protection against respiratory pathogens.
a, b, Lung damage was assessed in the BALF of X31 influenza virus infected WT (n = 8) and Cyp1a1/Cyp1b1 double-knockouts (Cyp1a2+/−) (n = 9) (a) or Cal09 H1N1 influenza virus infected WT (n = 6) and Cyp1−/− (n = 5) mice (b) by measurement of total cells, Ter119+ RBCs, total protein, and serum albumin concentrations on day 6 post infection. (c) Weight loss of influenza (X31) and Streptococcus pneumoniae coinfected WT (n = 23) and Cyp1−/− (n = 23) mice. All Data are representative of two independent experiments or pooled from three experiments (c). Statistical analysis was performed using unpaired two-tailed Student’s t test (a, b) or two-way ANOVA with Sidak’s post-test (c) and significant P values are indicated on the graphs. Data are shown as mean±SEM.
Source data
Extended Data Fig. 5 Endothelial-specific AHR deletion.
a, Endothelial-specific AHR deletion was determined by measuring expression of Ahr and AHR-target gene Cyp1a1 in isolated lung CD31+ endothelial cells in Cdh5Cre-ERT2Rosa26-LSL-YFP; Ahrflox/flox mice (ECΔAhr) and WT control (Cdh5Cre−Rosa26-LSL-YFP; Ahrflox/flox) mice by qPCR (n = 8) (a) or transcripts per million (TPM) from bulk RNA-seq analysis (n = 3) (b). c, YFP-expressing lung endothelial cells as a measurement of Cre induction was determined in CD31+ lung endothelial cells by flow cytometry in ECΔAhr mice (n = 5). All Data are representative of two independent experiments. Statistical analysis was performed using unpaired two-tailed Student’s t test and significant P values are indicated on the graphs. Data are shown as mean±SEM.
Source data
Extended Data Fig. 6 AHR deletion in endothelial cells does not drastically alter influenza-induced pulmonary inflammation.
a, b Immune cell numbers were determined in the BALF of WT (n = 6) and ECΔAhr (n = 5) mice (a) and whole lung (n = 4) (b) of on day 6 post infection by flow cytometry. c, BALF cytokine concentration in WT and ECΔAhr mice was determined on day 2 (IFN) or day 6 (remaining cytokines) post infection (IL-6 and IFN-λ: WT n = 6, ECΔAhr n = 8; remaining cytokines n = 4). All Data are representative of two to three independent experiments. Statistical analysis was performed using unpaired two-tailed Student’s t test and significant P values are indicated on the graphs. Data are shown as mean±SEM. ns, not significant.
Source data
Extended Data Fig. 7 AHR signalling in endothelia prevents airway epithelial apoptosis and dysplastic repair.
a, b, Heatmaps of indicated genes from bulk RNA sequencing data comparing naïve WT and ECΔAhr CD31+ lung endothelial cells (a) and EpCam+ lung epithelial cells on day 6 post infection (b) (fold change > 1.5, padj < 0.05). c, Frequency (% of total EpCam+) and proliferation (Ki67+) of distal airway stem cells (EpCamhighCD24lowMHC-II–) in the lungs of WT and ECΔAhr mice was measured by flow cytometry in naïve (n = 3) mice and on day 6 post influenza infection (WT n = 6; ECΔAhr n = 5). d, Frequency of apoptotic (Annexin-V+) and necrotic (TO-PRO-3+) lung endothelial cells (CD31+), progenitor airway epithelial cells (EpCamhighCD24lowMHC-II–), mature airway epithelial cells (EpCamhighCD24highMHC-II–), and type II alveolar epithelial cells (EpCamlowMHC-II+) was assessed by flow cytometry in WT and ECΔAhr mice on day 6 post influenza infection (n = 4). All Data are representative of two independent experiments. Statistical analysis was performed using one-sided Wald test with Benjamini–Hochberg correction (a, b) or two-way ANOVA with Sidak’s post-test (c) and significant P values are indicated on the graphs. Data are shown as mean±SEM. ns, not significant.
Source data
Extended Data Fig. 8 AHR-dependent regulation of the apelin signalling pathway in lung endothelia.
a, Heatmap of indicated genes from RNA sequencing data comparing CD31+ lung endothelial cells on day 6 post infection (fold change > 1.5, padj < 0.05). b, Expression of indicated genes in isolated CD45+ immune cell, EpCam+ epithelial cell, and CD31+ endothelial cell was determined by qPCR in naïve WT mice (n = 4). c, d, Expression of indicated genes in UMAP plots of mouse (c) and human (d) lung scRNA-seq datasets obtained from lungendothelialcellatlas.com. e, Lung vascular leakage was assessed in PBS (n = 5) and apelin (n = 6) treated ECΔAhr mice by quantification of total cells Ter119+ RBCs in the BALF on day 6 post infection. f, Dot plot of hallmark pathways enriched or downregulated in MM54-treated WT mice (relative to PBS-treated controls) by GSEA. Comparisons are between MM54-treated and untreated from influenza infected mice, for endothelia and epithelia (two pairwise comparisons total). Dot size relates to statistical significance. All Data are representative of at least two independent experiments. Statistical analysis was performed using one-sided Wald test with Benjamini–Hochberg correction (a) or followed by Tukey’s post-test (b), or unpaired two-tailed Student’s t test (e). NES were generated with GSEA using a two-sided Kolmogorov Smirnov statistic with Hallmark genesets on genelists ranked by the Wald t statistic from DESeq2 (f) and significant P values are indicated on the graphs. Data are shown as mean±SEM. ns, not significant.
Source data
Extended Data Fig. 9 Dietary AHR ligands do not disrupt pulmonary inflammation.
a, Immune cell numbers were determined in the whole lung of WT mice fed purified or I3C-enriched diet on day 6 post infection by flow cytometry (n = 4). b, BALF IFN (day 2) and cytokine (day 6) concentrations in WT mice fed purified or I3C-enriched diet (n = 5). Statistical analysis was performed using unpaired two-tailed Student’s t test and significant P values are indicated on the graphs. Data are shown as mean±SEM. ns, not significant.
Source data
Supplementary information
Supplementary Information
This file contains a guide to Supplementary Tables 1–4 (table files supplied separately).
Reporting Summary
Supplementary Table 1
Supplementary Table 2
Supplementary Table 3
Supplementary Table 4
Peer Review File
Source data
Source Data Fig. 1
Source Data Fig. 2
Source Data Fig. 3
Source Data Fig. 4
Source Data Extended Data Fig. 2
Source Data Extended Data Fig. 3
Source Data Extended Data Fig. 4
Source Data Extended Data Fig. 5
Source Data Extended Data Fig. 6
Source Data Extended Data Fig. 7
Source Data Extended Data Fig. 8
Source Data Extended Data Fig. 9
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and Permissions
About this article
Cite this article
Major, J., Crotta, S., Finsterbusch, K. et al. Endothelial AHR activity prevents lung barrier disruption in viral infection.
Nature (2023). https://doi.org/10.1038/s41586-023-06287-y
-
Received: 02 June 2022
-
Accepted: 06 June 2023
-
Published: 16 August 2023
-
DOI: https://doi.org/10.1038/s41586-023-06287-y
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.