Cardiovascular
Photonic skin sensing network for cardiovascular health monitoring
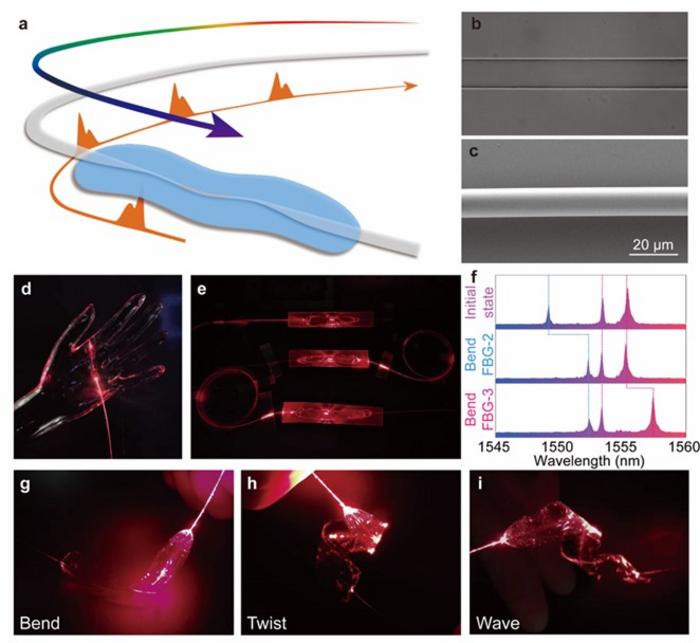
image: Figure 1. Skin-like microfiber Bragg grating (μFBG) patch.
view more
Credit: OEA
A new publication from Opto-Electronic Advances, 10.29026/oea.2023.230018 discusses Spatiotemporal hemodynamic monitoring via configurable skin-like microfiber Bragg grating group.
Cardiovascular disease is the world’s leading cause of death. According to the World Health Organization, 17.9 million people die every year due to cardiovascular diseases. For the prewarning and accurate treatment of cardiovascular diseases, it is important to monitor hemodynamic parameters continuously, including blood pressure (BP), heart rate (HR), peripheral resistance (PR), and vascular elasticity. Soft wearable devices are well suited to monitoring physiological signals such as electrocardiogram (ECG) signals, phonocardiogram (PCG) signals, and pulse waves with the advantages of real-time operation capability, skin-like mechanical properties, and high-SNR sensing capability. However, the human cardiovascular system is complicated and distributed with network circulation. Monolithic hemodynamic parameters achieved by current wearable devices cannot adequately and precisely reflect the health status of regional vasculature. A spatiotemporal hemodynamic monitoring technique is urgently needed to satisfy the ever-growing demand for clinical treatment and daily health management of the cardiovascular system.
The distributed optical fiber (DOF) sensing technique represented by the fiber Bragg grating (FBG) is ideally suited for spatiotemporal hemodynamic monitoring. Its spatially distributed multichannel sensing capability, excellent temporal synchronization and lack of electromagnetic interference lay a foundation for multiple high-SNR physiological signal monitoring. However, the traditional optical fiber has a large-distinct mechanical property with the skin and a low response on physiological signals considering its rigid and brittle silica material and thick diameter of 125 μm, making it difficult to be worn on the body stably and comfortably. Flexible packaging technology has been used to address the mechanical mismatch. Nevertheless, excessive thick encapsulation and the low sensitivity of commercial FBG devices pose an obstacle in detecting subtle physiological signals, thereby limiting their potential applications in wearable devices. Optical microfibers have been proven to have excellent flexibility, configurability, and large evanescent fields for high sensitivity sensing. However, the existing devices based on optical microfiber are difficult to achieve spatially distributed, time-synchronized, and multi-parameter sensing capabilities without a wavelength encoding strategy.
The authors of this article propose a spatiotemporal hemodynamic monitoring technique based on a skin-like microfiber grating group. The technique employs microfiber and ultra-thin flexible packaging technology to prepare skin-like microfiber patches. By effectively reducing the equivalent modulus of the device and the cross-sectional area of the microfiber, the stress response of the patch is improved by 2 orders of magnitude (the sensitivity is 5.26 nm/N under a stress within 50 mN). It also shows great repeatability and stability under 10,000 stress circles. In addition, the technique employs femtosecond laser direct writing technology to non-invasively inscribe Bragg gratings into the interior of the microfiber, providing different wavelength encodings for multiple microfiber patches, enabling the synchronous multi-channel sensing capabilities. By connecting microfiber grating (μFBG) patches in series, multiple physiological signals at different nodes of the human body can be detected simultaneously and distinguished by different working wavelengths. Since the light-based physiological signals propagate at close to the speed of light in the μFBG group, the time synchronization is only limited by the FBG interrogator. By detecting the proximal ballistocardiograph (BCG) signal and the distal pulse wave at each superficial artery in the human cardiovascular system, and then calculating the pulse wave transmit time (PTT), the spatiotemporal hemodynamics monitoring technology is established.
By detecting mechanical signals at the proximal and distal ends of the cardiovascular system instead of electrophysiological activity signals, the monitoring technique can present the real dynamics of the systemic cardiovascular system, such as heartbeat, angiectasis, and pulse wave propagation. Three hemodynamic monitoring modes are presented in this study. First, pulse waves of different superficial arteries in the human body, such as carotid artery, radial artery and pedal artery, were collected, and PTTs were analyzed using the BCG signal. Different PTTs arise from the differences in the length, diameter, and elastic modulus of blood vessels. This mode could enable the health assessment of local arterial branches in the cardiovascular system. Second, the μFBG group dynamically recorded the subjects’ dual-channel physiological signals during the process of exercise and rest. The heart rate was calculated by the cardiac cycle, and the pulse wave propagation velocity changed along with the blood pressure. In addition, the μFBG group dynamically recorded the dual-channel physiological signals when the external pressure imposed, and the changes of PTT could sensitively reflect the different degrees of peripheral arterial resistance. This real-time local peripheral vascular resistance monitoring technique was proposed for the first time.
This study develops the synchronous multi-channel sensing technology based on the skin-like μFBG group, which has significant advantages such as temporal dynamic, spatial distribution, easy networking and configurability, high sensitivity and high flexibility. The proposed spatiotemporal hemodynamic monitoring technology has the working capability of real-time and dynamic evaluation of local blood vessel health status in the whole cardiovascular system, demonstrating the great potential in the diagnosis of cardiovascular diseases such as arrhythmia, angiosclerosis, hypertension, and thrombosis and facilitating precise clinical diagnosis, the fast screening of lesions, and daily health management.
# # # # # #
Dr. Fei Xu is currently a professor at the College of Engineering and Applied Sciences, Nanjing University, China. He is a winner of the National Science Foundation for Distinguished Young Scholars (2019) from the National Natural Science Foundation of China. He is also the Fellow of SPIE and the Senior Member of Optica/IEEE. He focuses his research on optical and electrical intelligent sensing technologies. To date, he has authored or coauthored 9 book chapters, > 20 invited reviews, granted >50 patents (China and abroad), granted 4 PCT and US patents, and > 160 peer-reviewed articles in academic journals including Science Advances, Optica, Light: Science & Application, Advanced Photonics, Advanced Materials, and etc. in the previously mentioned areas.
Professor Fei Xu’s home page: https://eng.nju.edu.cn/xf/main.htm
With optical fiber as the carrier, the laboratory has independently developed optical fiber integrated devices and systems with ultra-high precision and excellent performance after over ten years accumulated high-precision processing technologies such as micro/nano processing technologies and femtosecond laser processing technologies, and developed the frontier applications in aerospace, medical diagnosis, optical fiber communication, safety monitoring, biochemical analysis, and other fields. With the support of major national requirements and key projects, the laboratory focuses on scientific issues in next-generation optical fiber integration, human-computer interaction and computational sensing, and innovates in advanced optoelectronic devices, integration methods, as well as algorithms and architectures of novel models through cross-merging physical optics, materials science, electronics, machine learning, micro/nano optics, and computer vision.
# # # # # #
Opto-Electronic Advances (OEA) is a high-impact, open access, peer reviewed monthly SCI journal with an impact factor of 8.933 (Journal Citation Reports for IF2021). Since its launch in March 2018, OEA has been indexed in SCI, EI, DOAJ, Scopus, CA and ICI databases over the time and expanded its Editorial Board to 36 members from 17 countries and regions (average h-index 49).
The journal is published by The Institute of Optics and Electronics, Chinese Academy of Sciences, aiming at providing a platform for researchers, academicians, professionals, practitioners, and students to impart and share knowledge in the form of high quality empirical and theoretical research papers covering the topics of optics, photonics and optoelectronics.
# # # # # #
More information: http://www.oejournal.org/oea
Editorial Board: http://www.oejournal.org/oea/editorialboard/list
All issues available in the online archive (http://www.oejournal.org/oea/archive).
Submissions to OEA may be made using ScholarOne (https://mc03.manuscriptcentral.com/oea).
ISSN: 2096-4579
CN: 51-1781/TN
Contact Us: oea@ioe.ac.cn
Twitter: @OptoElectronAdv (https://twitter.com/OptoElectronAdv?lang=en)
WeChat: OE_Journal
# # # # # #
Zhu HT, Luo JX, Dai Q, Zhu SG, Yang H et al. Spatiotemporal hemodynamic monitoring via configurable skin-like microfiber Bragg grating group. Opto-Electron Adv 6, 230018 (2023). doi: 10.29026/oea.2023.230018
# # # # # #
Journal
Opto-Electronic Advances