Congenital disorders
Analysis of the benefit of gonadotropin-releasing hormone agonist treatment in premenopausal women undergoing hematopoietic cell transplantation
Abstract
Gonadotropin-releasing hormone agonist (GnRHa) appears to exhibit ovarian protection during chemotherapy for malignant tumors. The purpose of this study was to analyze the benefits of GnRHa in premenopausal women undergoing hematopoietic cell transplantation (HSCT). Candidates for myeloablative chemotherapy HSCT requiring fertility preservation in the Gynecological Endocrinology Clinic of Peking University People’s Hospital from December 2011 to December 2021 were retrospectively analyzed. Patients who chose to receive GnRHa treatment were given at least 2 courses of a 3.75-mg dose of a GnRHa before myeloablative chemotherapy, and patients who chose not to receive GnRHa treatment were included in the control group. All patients were monitored for menstruation return and menopause-related symptoms, and ovarian function tests [follicle-stimulating hormone (FSH), luteinizing hormone, and estradiol] were performed 6–12 months after HSCT. In addition, we assessed the vaginal bleeding of patients in the laminar air-flow room (LAFR). A total of 234 cases were included in this study: 77 cases in the treatment group and 157 cases in the control group. The incidence of vaginal bleeding in the LAFR in the treatment group was significantly lower than that in the control group (24.68% vs. 79.62%, P < 0.001). The menopausal symptoms of the patients in the treatment group were reduced after transplantation (46.75% vs. 19.75%, P < 0.001). There was no difference in visible follicles by follow-up ultrasound in the two groups after HSCT (16.88% vs. 13.38%, P = 0.474). The level of FSH at 6–12 months after transplantation was lower (98.00 mIU/ml vs. 117.53 mIU/ml, P = 0.001). The proportion of patients with FSH < 40 mIU/ml did not differ between the two groups. One patient in the treatment group recovered spontaneous menstruation, while none recovered spontaneous menstruation in the control group (1.30% vs. 0%, P = 0.329). The use of GnRHa may relieve menopause-related symptoms and reduce vaginal bleeding in the LAFR and breakthrough bleeding after transplantation. GnRHa treatment can reduce the level of FSH after myeloablative chemotherapy, but it cannot reduce the incidence of premature ovarian failure in women of reproductive age following myeloablative HSCT.
Introduction
Hematopoietic stem cell transplantation (HSCT) is a well-established treatment for many congenital or acquired diseases of the hematopoietic system and several other life-threatening conditions1 that can effectively improve the survival rate of patients. With the advancement of medical technology, the long-term survival rate of such patients has increased significantly2. An increasing number of people are pursuing quality of life on the basis of surviving, and have the need to establish a family or fertility needs. Huang and colleagues at Peking University established the “Beijing Protocol” for haplo-SCT using myeloablative conditioning (MAC) regimen3, solving the problem of insufficient hematopoietic stem cell donors. Patients with nonhematological malignancies (such as severe aplastic anemia) or patients who are older than 55 years or exhibit impaired vital organ function can choose the reduced intensity conditioning (RIC) regimen. However, the commonly used MAC regimens and RIC regimen both include high-dose chemotherapy and total-body irradiation. The chemotherapy dosages used in these combinations are very gonadotoxic. It has been reported that the prevalence rate of ovarian insufficiency exceeds 90–100% of female patients who have received HSCT following MAC4,5,6. Our team’s single-center preliminary study found that the incidence of premature ovarian failure (POF) after HSCT in female patients over 20 years old can reach 100%7. Studies have shown that HSCT is an independent risk factor for POF8.
Although impaired ovarian function after HSCT is a clinically recognized phenomenon, there are few studies on the protection of ovarian function, with most of these investigations focusing on the cryopreservation of oocytes, embryos, and ovarian tissues9, 10. These studies partially address fertility issues associated with ovarian failure but do not apply to patients who must undergo transplants in the short term or who are physically or financially disadvantaged.
Due to iatrogenic POF, patients also face the early onset of menopausal symptoms, which affects quality of life. Moreover, patients with hematologic disorders may face more severe abnormal uterine bleeding in the laminar air-flow room (LAFR)11, 12. In the event of uncontrollable severe vaginal bleeding, the patient’s life may be in danger, eventually leading to treatment failure. Currently, the commonly used treatment to stop bleeding in the LAFR is mainly comprises high doses of hormone-based drugs, such as norethindrone, combined oral contraception (COC). However, there is relatively little awareness of the prevention of bleeding in the LAFR.
Gonadotropin-releasing hormone agonist (GnRHa) use in gynecological endocrine therapy can put patients into a pseudomenopausal state. In recent years, the protective effect of GnRHa on the ovary during chemotherapy has attracted attention. Several meta-analyses and prospective randomized studies13, 14 have shown that GnRHa significantly reduces the risk of POF in women undergoing gonadotoxic chemotherapy. Although these results seem promising, a paucity of data exists on similar use of GnRHa in the HSCT population, especially in China. Several small studies have suggested an inconclusive benefit of using a GnRHa to preserve ovarian function in the HSCT patients. If GnRHa therapy works for HSCT patients, it will serve as an economical, noninvasive and simple method to protect ovarian function. Therefore, this study analyzed the benefits of GnRHa treatment in premenopausal women undergoing HSCT, including the reduction of vaginal bleeding in the LAFR, protection of ovarian function, and improvement in perimenopausal symptoms.
Methods
This was a retrospective cohort study. We affirm that: (1) the study was approved by the Ethics Committee of Peking University People’s Hospital (No. 2015PHB087-01), including any relevant details; and (2) all experiments were performed in accordance with relevant guidelines and regulations. Moreover, all research was performed in accordance with relevant regulations, and appropriate consent was obtained from all participants and/or their legal guardians and signed by the patients or their families. Patients with hematological diseases at Peking University People’s Hospital routinely undergo gynecological physical examination before HSCT, such as the collection of menstrual history, pelvic examination and cervical cancer screening. Patients with a desire for fertility protection are triaged to the gynecological endocrinology clinic for consultation on fertility protection methods. The research subjects of this study were all from the gynecological endocrinology clinic.
Each patient requiring fertility preservation in the gynecological endocrinology clinic was informed by gynecologists about methods of ovarian protection, and was fully informed that the efficacy of GnRHa was not clear. Patients could choose whether to use GnRHa voluntarily, and signed the informed consents. Patients chose to use GnRHa were injected leuprolide acetate 3.75 mg subcutaneously before the start of myeloablative chemotherapy, once every 28 days as a course of treatment. Considering the onset time and safety of GnRHa, patients were advised to get at least two injections of GnRHa before entering LAFR. The gynecological endocrinologists kept the medical records of all patients who planned to undergo HSCT whether GnRHa was used, and reviewed the treatment process of the patients through the medical records.
The clinical data of all patients were collected for analysis when they went returned to the gynecological endocrinology clinic 6–12 months after HSCT. The patients were followed up by assessing vaginal bleeding in the LAFR, menstruation return, menopause-related symptoms, and ovarian function [follicle-stimulating hormone (FSH), luteinizing hormone (LH), and estradiol] were done.
We reviewed data from all HSCT patients for whom medical records were collected in clinic. The study population consisted of women of childbearing age under 40 years of age who were scheduled to undergo myeloablative chemotherapy before HSCT from December 2011 to December 2021. Patients who voluntarily chose to inject GnRHa were included in the treatment group. Patients who chose not to receive GnRHa treatment were included in the control group. The inclusion criteria were as follows: (1) women undergoing myeloablative chemotherapy HSCT for hematological diseases; (2) age < 40 years; and (3) patients with normal hormone levels or without laboratory data but with regular menstruation before HSCT. The exclusion criteria were as follows: (1) patients whose ovarian function was found to have declined before HSCT with myeloablative chemotherapy through menstruation, B-ultrasound monitoring and hormone examination; (2) patients who were unable to survive after HSCT after myeloablative chemotherapy; (3) patients with incomplete clinical data (including patients who were not followed up within 1 year after HSCT); and (4) patients who received less than 2 courses of GnRHa treatment before myeloablative chemotherapy;and (5) patients who were lost to follow-up (more than 1 year after HSCT).
POF was defined as age 10 Gy); carmustine, etoposide, cytarabine, and melphalan; and a single melphalan dose > 140 mg/m2.
All data were statistically processed using the SPSS 25.0 (SPSS Inc., Chicago, IL) statistical software package. Continuous variables are herein were described using medians, ranges and ranges; categorical variables are described using frequencies and proportions. Comparisons were performed using the chi-square test, Fisher’s exact test, and Mann–Whitney U test. P < 0.05 indicated that the difference was statistically significant.
Results
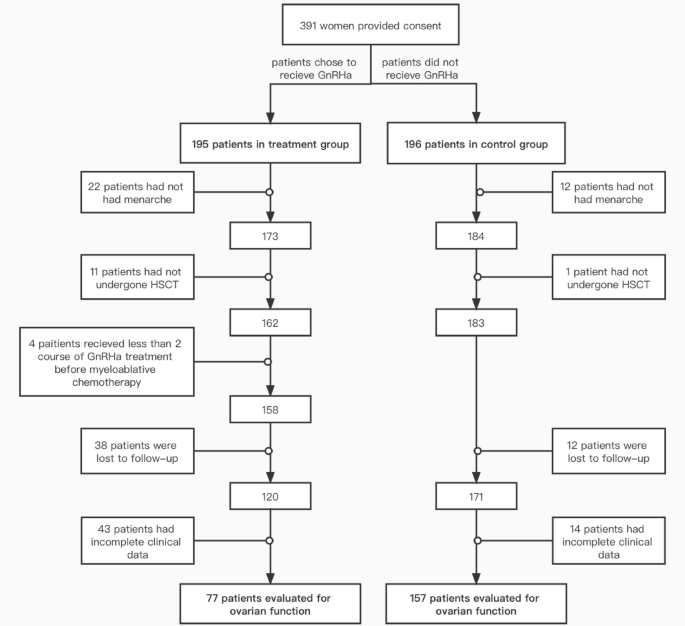
From December 2011 to December 2021, 391 patients visited Peking University People’s Hospital Gynecology Clinic for fertility protection counseling before undergoing myeloablative chemotherapy and HSCT. A total of 234 cases were included in this study, followed up for a median of 28 months. There were 77 cases in the treatment group and 157 cases in the control group (Fig. 1). The general conditions and clinical data of the study population are shown in Table 1. All patients underwent allogeneic transplantation. Except for AA patients who received RIC regimen, the rest of the patients all used MAC regimen. There were significant differences between the two groups in terms of the hematologic disease type and whether they received cyclic chemotherapy before myeloablative chemotherapy and HSCT. Compared with the control group, the treatment group was less likely to receive courses of chemotherapy before myeloablative chemotherapy and HSCT (67.53% vs. 85.35%, P = 0.002). There were no statistically significant differences between the two groups in terms of age, HLA- matching status, or graft vs. host disease (GVHD).
Flow sheet explaining patient dropout. HSCT hematopoietic stem cell transplantation, GnRHa
gonadotropin-releasing hormone agonist.
The median follow-up times for the treatment and control groups were 28 and 29 months, respectively. The benefits of patients in the treatment group are mainly to reduce the rate of vaginal bleeding in LAFR and reduce menopausal symptoms. The incidence of vaginal bleeding in the LAFR, in the treatment group was significantly lower than that in the control group (24.68% vs. 79.62%, P < 0.001). Compared with those in the control group, the menopausal symptoms of the patients in the treatment group were reduced after transplantation (46.75% vs. 19.75%, P < 0.001) (Table 2).
Hormone levels were measured before HSCT in 35 of 77 patients in the GnRHa group and in 20 of 157 patients in the control group. The data showed no difference between FSH levels and LH levels between the two groups before HSCT (5.69 mIU/ml vs. 5.93 mIU/ml, P = 0.582, 5.15 mIU/ml vs. 4.96 mIU/ml, P = 0.564). The medians of E2 levels were 36.32 pg/ml and 26.38 pg/ml. Observation indices between the treatment group and control Group 6–12 months after HSCT are shown in Table 1. There was no difference in visible follicles by the follow-up ultrasound in the two groups of patients after HSCT (16.88% vs. 13.38%, P = 0.474). The level of FSH at 6–12 months after transplantation was lower in the treatment group (98.00 mIU/ml vs. 117.53 mIU/ml, P = 0.001), although both levels had reached the POF standard. There was no significant difference in LH levels after transplantation (65.07 mIU/ml vs. 61.38 mIU/ml, P = 0.127). Because the sensitivity of the E2 assay was 20 pg/ml, most of these levels were not detectable as they were 20 pg/ml or lower. However, the median E2 level after HSCT in both groups was less than 20 pg/ml. The proportion of patients with FSH < 40 mIU/ml did not differ between the two groups. Only one patient in the treatment group recovered spontaneous menstruation while none in the control group did (1.30% vs. 0%, P = 0.329). That patient was a 25-year-old acute lymphocytic leukemia patient who returned to a regular menstrual cycle with normal hormone levels (Table 2).
Whether or not to perform pretransplant cyclic chemotherapy was associated with the type of hematological disease, and the conclusion remained unchanged after we stratified the main outcome indicators according to the type of disease (Supplementary material).
Discussion
Our study showed that the use of a GnRHa before transplantation did not preserve ovarian function in patients who underwent HSCT using myeloablative regimens.
Chemotherapy-induced POF was first reported in the late 1950s15. Subsequent studies suggested that the effect of ovarian damage may be age dependent and dose dependent16. The “Beijing Protocol” using the MAC regimen improved the success rate of allogeneic haplo-SCT, which also meant that high-dose chemotherapy and total-body irradiation were used. The usual MAC regimen for allogeneic HSCT usually includes high-dose alkylating chemotherapy drugs such as cyclophosphamide, and/or total body irradiation (TBI). The commonly used MACs are the classic TBICy and BuCy regimens and their improved regimens. The classic TBICy and BuCy regimens both include cyclophosphamide 120 mg/kg for 2 days and 12–14 Gy TBI for 2 weeks or busulfan 12.8 mg/kg for 4 days. The dose of cyclophosphamide in the improved regimen is 3.6 g/m2 for 2 days, and the radiotherapy regimen is a single 770 cGy TBI. Even though the RIC regimen reduces the dose of cyclophosphamide to 90–120 mg/kg for 2–4 days, the dose of chemotherapy drugs used in the regimen is still much higher than that of conventional chemotherapy. Compared with the dose of a course of chemotherapy (for example, the dose of cyclophosphamide in conventional chemotherapy for breast cancer is 0.5 mg/m2), the chemotherapy dose in the conditioning regimen will cause more serious damage to the ovary. Treatment-induced POF is a major problem for surviving women of reproductive age. Nakayama et al.17 conducted a survey and reported that most patients believed that a discussion of fertility-related or menopausal-related issues was as important as a discussion of their cancer issues and suggested that health care providers should provide information on fertility and menopause repeatedly throughout the treatment period, and that menopause-related information should be reemphasized after HSCT. The American Society of Clinical Oncology guidelines recommend the discussion of ovarian preservation as early as possible in treatment planning18.
GnRHa drugs are synthetic peptide drugs modeled on GnRH that are designed to interact with the GnRH receptors and modify the release of gonadotropins. The protective mechanisms of GnRHa drugs on ovarian function remain unclear. Several proposed mechanisms for how GnRHa therapy works have been proposed; these include GnRHa suppression of can suppress gonadotropin levels to stimulate the prepubertal hormonal milieu, which subsequently prevents primordial follicle maturation, reducing the number of follicles susceptible to chemotherapy19. Alternatively, a GnRHa can reduce utero-ovarian perfusion, resulting in less exposure of the ovaries to chemotherapeutic agents20. Alternatively, it can directly activate GnRH receptors on the ovary and regulate the expression of anti-apoptotic molecules in the gonads21. GnRHa therapy has been shown to preserve ovarian function in chemotherapy-treated patients outside of the HSCT setting in meta-analysises and prospective randomized studies13, 14, 22. Blumenfeld et al.23 presented their meta-analysis of 20 studies (15 retrospective and 5 randomized, controlled trials) that have reported on 1837 patients treated with a GnRHa in parallel with chemotherapy, showing a significant decrease in the POF rate in survivors. In 2015, Moore et al.24 published the results of a prospective RCT trial, in which 257 premenopausal breast cancer patients received chemotherapy with or without GnRHa treatment, showing that GnRHa-treated patients had better-preserved ovarian function across multiple endpoints than the controls. However, there are few data on the protection of ovarian function by GnRHa treatment in the HSCT population. Cheng et al.4 conducted a prospective phase II study to evaluate the efficacy of a GnRHa in reducing the incidence of POF in the setting of HSCT. Seven of 44 patients (16%) regained ovarian function in the study and they concluded that the use of GnRHa before transplantation did not preserve ovarian function in HSCT patients receiving either myeloablative or nonmyeloablative regimens. However, the study did not include patients who were not injected with GnRHa as controls.
Our study showed that GnRHa treatment applied before myeloablative chemotherapy may be able to reduce the FSH level of patients after HSCT, but it still reaches the standard of POF; this finding revealed that GnRHa cannot reduce the incidence of premature ovarian failure in patients receiving myeloablative chemotherapy HSCT. Only one case maintained spontaneous menstruation, and those hormone levels suggested normal ovarian function, while no such cases were observed in the control group. In conclusion, GnRHa treatment could not reduce the incidence of premature ovarian failure. Combined with previous studies to analyze this study, the following aspects deserve attention. In terms of diagnosis, the diagnostic criteria for POF in this study were age < 40 years old and FSH ≥ 40 mU/ml. However, cyclic HRT was given when the hormone levels reached the POF standard 6–12 months after HSCT, especially in patients with perimenopausal symptoms. In terms of the timing of medication, all patients in this study were given GnRHa before the initiation of MAC. However, considering that some patients with hematological malignancies in the study population received unprotected, gonadotoxic chemotherapy before GnRHa was administered, a stratified analysis was performed. The results showed that the application of a GnRHa did not significantly reduce the incidence of premature ovarian failure, regardless of whether the group was based on disease type or whether there was chemotherapy before MAC.
Although GnRHa treatment does not reduce the incidence of premature ovarian failure, there are other benefits for female patients who use GnRHa before HSCT. The results showed that of GnRHa injections at least 2 courses before transplantation were effective in reducing vaginal bleeding during transplantation. Other studies have also shown that GnRHa treatment may prevent breakthrough bleeding post-transplantation25. Once a donor is identified, the recipient completes a pretransplant evaluation and then undergoes a conditioning regimen (1–2 weeks), receives the graft infusion, and then must await the initial signs of engraftment (10–28 days) and repopulation of bone marrow (60–90 days)26. Myeloablative conditioning typically results in severe pancytopenia within 1–3 weeks of initiation, and thrombocytopenia increases the risk of heavy menstrual bleeding; potentially life-threatening heavy menstrual bleeding may delay treatment, leading to suboptimal outcomes. Therefore, the menstrual management of patients in the LAFR cannot be ignored. Even without regard to ovarian protection, GnRHa therapy requires only a simple injection and has few side effects when given regularly over 2 courses before pancytopenia, which makes it the first choice of many transplant physicians. Therefore, GnRHa injection before HSCT can be recommended for women of childbearing age to prevent vaginal bleeding during bone marrow transplantation and breakthrough bleeding after transplantation.
Menopause-related symptoms are also important reference indicators for evaluating the ovarian protective function of GnRHa drugs. Previous studies have shown that women with premature ovarian failure who undergo HSCT have lower scores on the symptomatic menopause rating scale and lower modified Kupperman index values than naturally postmenopausal women of the same number of years after menopause27. The five most frequently reported perimenopausal symptoms were recorded by the gynecological clinician at the patient’s follow-up visit after transplantation. Although GnRHa treatment did not yield a benefit of ovarian protection in terms of diagnostic criteria based on hormone levels, such therapy can significantly reduce the incidence of perimenopausal symptoms; moreover, the role of GnRHa treatment in ovarian protection is difficult to deny, although further research is needed to prove it.
This study has limitations. First of all, it was not a randomized controlled trial (RCT). The patients’ exposure history could only be reviewed through medical records, and laboratory examination records were incomplete. This also leads to a high dropout rate of patients due to the loss of follow-up and incomplete clinical data. Second, the final sample size available for analysis in this study was relatively small. Differences in the number of patients with various diseases and differences in the treatment plan and length of treatment before HSCT all increased the possible bias.
Conclusion
In conclusion, the benefits of GnRHa thrapy can be seen in reducing vaginal bleeding and menopausal symptoms in women of childbearing age who receive myeloablative HSCT. GnRHa drugs may decrease FSH levels after myeloablative chemotherapy, which may suggest that GnRHa treatment has a certain ovarian protective effect but does not reduce the incidence of POF. The clinical application value of GnRHa in fertility-sparing treatment of female patients of reproductive age who are undergoing myeloablative HSCT still needs to be validated by more standardized and rigorously designed RCTs with a larger number of included study populations. However, standardized injection of GnRHa can be considered as a way to prevent vaginal bleeding in the LAFR and improve menopausal symptoms after iatrogenic POF.
Data availability
The datasets used and analysed during the current study available from the corresponding author on reasonable request.
References
-
Xu, L. et al. The consensus on indications, conditioning regimen, and donor selection of allogeneic hematopoietic cell transplantation for hematological diseases in China-recommendations from the Chinese Society of Hematology. J. Hematol. Oncol. 11(1), 33 (2018).
Google Scholar
-
Zeng, Y. et al. Optimal donor for severe aplastic anemia patient requiring allogeneic hematopoietic stem cell transplantation: A large-sample study from China. Sci. Rep. 8(1), 2479 (2018).
Google Scholar
-
Lv, M., Chang, Y. J. & Huang, X. J. Update of the “Beijing Protocol” haplo-identical hematopoietic stem cell transplantation. Bone Marrow Transplant. 54(Suppl 2), 703–707 (2019).
Google Scholar
-
Cheng, Y. C., Takagi, M., Milbourne, A., Champlin, R. E. & Ueno, N. T. Phase II study of gonadotropin-releasing hormone analog for ovarian function preservation in hematopoietic stem cell transplantation patients. Oncologist 17(2), 233–238 (2012).
Google Scholar
-
Grigg, A. P., McLachlan, R., Zaja, J. & Szer, J. Reproductive status in long-term bone marrow transplant survivors receiving busulfan-cyclophosphamide (120 mg/kg). Bone Marrow Transplant. 26(10), 1089–1095 (2000).
Google Scholar
-
Somali, M. et al. Function of the hypothalamic–pituitary–gonadal axis in long-term survivors of hematopoietic stem cell transplantation for hematological diseases. Gynecol. Endocrinol. 21(1), 18–26 (2005).
Google Scholar
-
Su, H. et al. Gynecological complications in long-term survivors after allogeneic hematopoietic cell transplantation-a single-center real-life cross-sectional study. Front. Med. (Lausanne) 9, 956867 (2022).
Google Scholar
-
Cattoni, A. et al. Hormonal replacement therapy in adolescents and young women with chemo- or radio-induced premature ovarian insufficiency: Practical recommendations. Blood Rev. 45, 100730 (2021).
Google Scholar
-
Joshi, S. et al. Clinical guide to fertility preservation in hematopoietic cell transplant recipients. Bone Marrow Transplant. 49(4), 477–484 (2014).
Google Scholar
-
Oktay, K. et al. Fertility preservation in patients with cancer: ASCO clinical practice guideline update. J. Clin. Oncol. 36(19), 1994–2001 (2018).
Google Scholar
-
Meirow, D. et al. Prevention of severe menorrhagia in oncology patients with treatment-induced thrombocytopenia by luteinizing hormone-releasing hormone agonist and depo-medroxyprogesterone acetate. Cancer 107(7), 1634–1641 (2006).
Google Scholar
-
Yokoe, D. et al. Infection prevention and control in health-care facilities in which hematopoietic cell transplant recipients are treated. Bone Marrow Transplant. 44(8), 495–507 (2009).
Google Scholar
-
Blumenfeld, Z. & Evron, A. Preserving fertility when choosing chemotherapy regimens—The role of gonadotropin-releasing hormone agonists. Expert Opin. Pharmacother. 16(7), 1009–1020 (2015).
Google Scholar
-
Zong, X. et al. Effects of gonadotropin-releasing hormone analogs on ovarian function against chemotherapy-induced gonadotoxic effects in premenopausal women with breast cancer in China: A randomized clinical trial. JAMA Oncol. 8(2), 252–258 (2022).
Google Scholar
-
Galton, D. A., Till, M. & Wiltshaw, E. Busulfan (1, 4-dimethanesulfonyloxybutane, myleran); Summary of clinical results. Ann. N. Y. Acad. Sci. 68(3), 967–973 (1958).
Google Scholar
-
Bines, J., Oleske, D. M. & Cobleigh, M. A. Ovarian function in premenopausal women treated with adjuvant chemotherapy for breast cancer. J. Clin. Oncol. 14(5), 1718–1729 (1996).
Google Scholar
-
Nakayama, K. et al. Receiving information on fertility- and menopause-related treatment effects among women who undergo hematopoietic stem cell transplantation: Changes in perceived importance over time. Biol. Blood Marrow Transplant. 15(11), 1465–1474 (2009).
Google Scholar
-
Lee, S. J. et al. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J. Clin. Oncol. 24(18), 2917–2931 (2006).
Google Scholar
-
Blumenfeld, Z., Zur, H. & Dann, E. J. Gonadotropin-releasing hormone agonist cotreatment during chemotherapy may increase pregnancy rate in survivors. Oncologist 20(11), 1283–1289 (2015).
Google Scholar
-
Blumenfeld, Z. How to preserve fertility in young women exposed to chemotherapy? The role of GnRH agonist cotreatment in addition to cryopreservation of embrya, oocytes, or ovaries. Oncologist 12(9), 1044–1054 (2007).
Google Scholar
-
Clowse, M. E. B. et al. Ovarian preservation by GnRH agonists during chemotherapy: A meta-analysis. J. Women’s Health 18(3), 311–319 (2009).
Google Scholar
-
Del Mastro, L. et al. Effect of the gonadotropin-releasing hormone analogue triptorelin on the occurrence of chemotherapy-induced early menopause in premenopausal women with breast cancer: a randomized trial. JAMA 306(3), 269–276 (2011).
Google Scholar
-
Blumenfeld, Z., Katz, G. & Evron, A. ‘An ounce of prevention is worth a pound of cure’: The case for and against GnRH-agonist for fertility preservation. Ann. Oncol. 25(9), 1719–1728 (2014).
Google Scholar
-
Moore, H. C. et al. Goserelin for ovarian protection during breast-cancer adjuvant chemotherapy. N. Engl. J. Med. 372(10), 923–932 (2015).
Google Scholar
-
Ghalie, R. et al. Prevention of hypermenorrhea with leuprolide in premenopausal women undergoing bone marrow transplantation. Am. J. Hematol. 42(4), 350–353 (1993).
Google Scholar
-
Chang, K., Merideth, M. A. & Stratton, P. Hormone use for therapeutic amenorrhea and contraception during hematopoietic cell transplantation. Obstet. Gynecol. 126(4), 779–784 (2015).
Google Scholar
-
Su, H., Li, H., Yang, X., Wang, C. & Zhao, Y. Assessment of menopausal symptoms and quality of life in women with premature ovarian failure after hematopoietic stem-cell transplantation for hematologic diseases. Menopause 28(1), 65–69 (2020).
Google Scholar
Funding
This study was supported by the Capital clinical application characteristic study: The standardization of hormone therapy for the iatrogenic premature ovarian failure (Project no. Z1211070010120). The study was also supported by Roche Diagnostics: Dynamic study for the effects of chemotherapy and bone marrow transplantation on ovarian function in pre-adolescent hematological disease survivors (Project no.2018PHB085-01).
Author information
Authors and Affiliations
Contributions
R.H.: Data curation, formal analysis, investigation, writing-original draft preparation. Z.S.: Validation, investigation, writing- original draft preparation. H.L.: Data curation. C.W.: Methodology, data curation, supervision. L.Z.: Writing-reviewing and editing. X.Y.: Methodology, writing-reviewing and editing, funding acquisition.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary Tables.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
Reprints and Permissions
About this article
Cite this article
Han, R., Song, Z., Li, H. et al. Analysis of the benefit of gonadotropin-releasing hormone agonist treatment in premenopausal women undergoing hematopoietic cell transplantation.
Sci Rep 13, 14497 (2023). https://doi.org/10.1038/s41598-023-40778-2
-
Received: 09 December 2022
-
Accepted: 16 August 2023
-
Published: 04 September 2023
-
DOI: https://doi.org/10.1038/s41598-023-40778-2
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.