Infection
Characteristics and Treatment of L. monocytogenes infections
Xiaojiao Xu,1,2 Yuheng Shan,3 Yuying Cen,2,4 Jiahua Zhao,2,4 Xiaosa Yang,2,4 Rui Liu,1,2 Qingche Tan,2 Yubao Ma,2 Mianwang He,2 Jiatang Zhang,1,2,4 Fei Yang,2 Shengyuan Yu2
1School of Medicine, Nankai University, Tianjin, 300071, People’s Republic of China; 2Department of Neurology, The First Medical Centre, Chinese PLA General Hospital, Beijing, 100853, People’s Republic of China; 3Department of Neurology, Characteristic Medical Centre of People’s Armed Police Force, Tianjin, 300162, People’s Republic of China; 4Medical School of Chinese PLA, Beijing, 100853, People’s Republic of China
Correspondence: Jiatang Zhang; Fei Yang, Department of Neurology, The First Medical Centre, Chinese PLA General Hospital, No. 28, Fuxing Road, Beijing, 100853, People’s Republic of China, Tel +86 13601380322 ; Tel +86 13910973120, Email [email protected]; [email protected]
Purpose: Listeria monocytogenes infections are rare in the central nervous system (CNS) and frequently difficult-to-diagnose. Our goal is to assess CNS listeriosis patients’ clinical characteristics, diagnosis, treatment, and prognosis.
Patients and Methods: Patients with CNS listeriosis admitted to the Department of neurology, the first medical center of the Chinese PLA general hospital, were enrolled in this study from March 2018 to August 2022.
Results: This study analyzed eight adults, including five males and three females. The average age of onset was (50.25 ± 11.52) years. The clinical manifestations included fever, headache, altered mental status, vomiting, seizures, neck rigidity, hemiplegia and cranial nerve palsies. Cerebrospinal fluid (CSF) tests revealed intracranial hypertension, elevated cell count and protein concentration, and decreased glucose levels. The positive rates of blood and CSF culture were 40% and 28.57%, respectively. All patients underwent CSF metagenomic next-generation sequencing (mNGS), with a 100% positive rate and the specific read number 12– 20394. Magnetic resonance imaging (MRI) exhibited leptomeningitis, meningoencephalitis, and brain abscess, and no specific changes were discovered in two patients. All patients received antibiotic treatment, seven were cured, and one died.
Conclusion: Monitoring the proportion of monocytes in blood and mNGS results of CSF can play a crucial role in diagnosing pathogens. Early and sufficient application of two to three sensitive antibiotics with a BBB permeability of 20– 30% for at least 2– 3 months can significantly improve CNS listeriosis prognosis.
Keywords: Listeria monocytogenes, central nervous system, CNS, clinical characteristics, treatment, metagenomic next-generation sequencing, mNGS
Introduction
Listeria monocytogenes (L. monocytogenes) is a facultative anaerobic Gram-positive bacterium first isolated from humans by Nyfeldt in 1929.1,2 Soil, water, vegetables, fruits, milk products, raw meat, and refrigerated processed foods contain many L. monocytogenes, which can be responsible for human listeriosis.3,4 This bacterial infection can lead to clinical presentations ranging from asymptomatic infection or mild self-limiting gastroenteritis in immunocompetent patients to bacteremia or central nervous system (CNS) infections in immunocompromised individuals, which would be fatal.5 Moreover, CNS listeriosis primarily affects specific population segments, including neonates, pregnant women, and elderly and immunocompromised hosts, such as those taking medications that suppress cell-mediated immunity.2,6,7 Recent trends in organ transplantation and the use of immunosuppressants have led to a rise in the number of patients clinically diagnosed with listeriosis.8,9 However, there is currently no consensus concerning its diagnosis and therapy resulting in high disability and mortality.
In this study, we evaluated the clinical characteristics, laboratory tests, imaging features, diagnosis, antibiotic treatment, and outcomes of 8 cases of CNS listeriosis in adults. Proposing our experience in diagnosis and treatment of CNS listeriosis.
Materials and Methods
The data from 8 adults diagnosed with CNS listeriosis at the first medical center of the Chinese PLA general hospital from March 2018 to August 2022 were retrospectively analyzed. Neurolisteriosis was diagnosed based on blood culture, cerebrospinal fluid (CSF) culture, or metagenomic next-generation sequencing (mNGS) of the CSF with a compatible clinical manifestation.3 The inclusion criteria were patients aged 18 years or older who were clinically diagnosed as CNS listeriosis. Patients with incomplete follow-up data or complex multipathogen infections were excluded.
The onset age, gender, L. monocytogenes infection etiology, clinical characteristics, magnetic resonance imaging (MRI) features, peripheral blood and CSF findings, antibiotic treatment, and prognosis were analyzed.
Results
Clinical Characteristics and Examination
This study included 8 patients with CNS listeriosis, consisting of five males and three females, with an average onset age was 50.25±11.52 years. Five patients had a history of immunosuppression, of which case 1 was diagnosed as systemic lupus erythematosus (SLE) for 2 months and accepted glucocorticoid (GC) and Mycophenolate mofetil (MMF) treatment; case 4 diagnosed spindle cell tumor previously and received two chemotherapy treatments before onset; case 5 had a history of SLE and took MMF treatment for 11 years; patient 7 was polymyositis and received GC, methotrexate (MTX) and leflunomide (LEF) treatment for more than 10 years; and diabetes diagnosed during hospitalization for patient 8; while the remaining three patients lacked any evidence of immunosuppression. Only patient 4 consumed chilled food before the disease onset. All cases in our study exhibited fever, with a body temperature >38°C, of which patients 5, 7, and 8 even up to 40°C or above. Six patients had neck stiffness except for patients 1 and 6; five had headache and altered mental status; three had vomiting; and only case 5 had seizures during the disease course; patients 1 and 2 also had cranial nerve palsies, primarily involving III, V, VI, and VII cranial nerves; and patients 1 and 5 with hemiplegia and language disorder. Table 1 presents the clinical manifestations of our case series.
 |
Table 1 The Clinical Characteristics of the Patients |
Laboratory examinations revealed leukocytosis in patients 2, 3 and 8, elevated monocyte percentage in six patients other than cases 1 and 3, C-reactive protein (CRP) increased in all cases, Interleukin-6 (IL-6) increased in seven cases except for patient 7, five patients underwent blood culture, and patients 5 and 8 tested positive. Lumbar puncture was performed in all patients, and the average time from onset to lumbar puncture was 5.25 days. CSF examinations demonstrated intracranial hypertension in all cases, with five patients having intracranial pressure >330mmH20, the elevation of cell count was discovered in seven cases, and the glucose levels decreased in six patients, and protein concentration elevated in all patients. The CSF culture and mNGS positive rates were 28.57% (2/7) and 100% (8/8), respectively (Table 2 and 3).
 |
Table 2 Laboratory Test Results and Imaging Findings in the Patients |
 |
Table 3 The Microbiological Investigations in the Patients |
Magnetic resonance examination (MRI) of the brain revealed that 75% (6/8) of adults in this study had abnormalities. Patients 1 (Figure 1) and 5 (Figure 2) showed brain abscesses, and the lesions slightly shrunk after antibiotic treatment for patient 5 (Figure 2g–i), while patient 1 died due to cerebral hernia. Cases 2 (Figure 3) and 4 (Figure 4) showed hydrocephalus and were alleviated after ventriculo-peritoneal drainage and lateral ventricular drainage, respectively. In addition, patients 3 (Figure 5) and 8 (Figure 6) presented leptomeningitis and meningoencephalitides, respectively, while there were no specific changes discovered in patients 6 and 7.
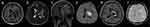 |
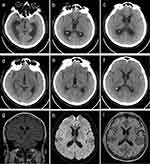
Figure 1 Brain magnetic resonance imaging of patient 1. Brain magnetic resonance imaging showed lesions in the left basal ganglia area with edema and midline shift (a–c). Restricted diffusion in the necrotic component on diffusion-weighted images (d), ring enhancing lesion on contrast-enhanced images (e), and mixed low signal in the necrotic component on susceptibility-weighted images (f). |
 |
Figure 2 Brain magnetic resonance imaging of patient 5. Brain magnetic resonance imaging performed 11 days after clinical onset showed lesions in the left frontal lobe with edema (a–c and f). Restricted diffusion in the necrotic component on diffusion-weighted images (d), and mild enhancement on contrast-enhanced images (e). The brain magnetic resonance imaging 1 month after clinical onset showed lesions in the left frontal lobe slightly shrunk (g–i). Diffusion-weighted images showed restricted diffusion in the necrotic center (j), ring enhancement on contrast-enhanced images (k), and ring low signal in the necrotic component on susceptibility-weighted images (l). |
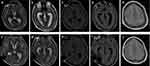 |
Figure 3 Brain magnetic resonance imaging of patient 2. Brain magnetic resonance imaging showed hydrocephalus (a and b) and leptomeningeal enhancement (c–e) 1 month after clinical onset. The hydrocephalus (f and g) and leptomeningeal enhancement (h–j) were slightly alleviated after ventriculo-peritoneal drainage and antibiotic treatment. |
|
Figure 4 Brain computed tomography and magnetic resonance imaging of patient 4. Brain computed tomography showed hydrocephalus 10 days after clinical onset (a–c). The hydrocephalus significantly relieved after lateral ventricular drainage (d–f). Brain magnetic resonance imaging performed 3.5 months after clinical onset showing no hydrocephalus (g–i). |
|
Figure 5 Brain magnetic resonance imaging of patient 3. Brain magnetic resonance imaging showed leptomeningeal enhancement (a–c) 5 days after clinical onset. 2 months after antibiotic treatment, the leptomeningeal enhancement was significantly alleviated (d–f). |
 |
Figure 6 Brain magnetic resonance imaging of patient 8. Brain magnetic resonance imaging performed 18 days after clinical onset showed abnormal signal of bilateral lateral ventricles (a and b) and leptomeningeal enhancement (c). |
Anti-Infective Therapy and Outcomes
The median time between the onset and initiation of treatment was 4.5 days. Five patients received empirical anti-tuberculosis treatment before identifying the pathogen. Targeted-antibiotic treatment was adjusted after confirming L. monocytogenes infections. Furthermore, patients 2 and 4 underwent ventriculoperitoneal drainage and lateral ventricular drainage due to hydrocephalus, respectively. In the end, five patients recovered completely, and patients 2 and 5 developed complications, while patient 1 died on the 15th day from the disease onset because of brain herniation (Table 4).
 |
Table 4 Summary of the Anti-Infective Therapies and Outcomes of the Patients |
Discussion
L. monocytogenes is ubiquitous in the natural environment; it can grow and reproduce in refrigeration temperatures, wide pH ranges, and high salt concentrations.2,8,10 Listeriosis is a rare and foodborne disease; approximately 99% of the cases are acquired by consuming L. monocytogenes-contaminated food.11–13 However, only one patient had chilled food stored in the refrigerator for a long time before the disease onset in this study. This bacterium is highly neurophilic and can result in CNS invasion in immunocompromised patients, causing meningitis, meningoencephalitis, rhombencephalitis, or brain abscess.10,12,14,15 However, CNS listeriosis can also occur in immunocompetent patients,16–18 and our study has three previously healthy patients. The listeriosis incidence rates have increased in developed countries yearly during the 21st century, and epidemiological investigation of listeriosis is lacking in China.2,19
The precise mechanisms by which L. monocytogenes infects the CNS remain a mystery. Pathogenesis may involve L. monocytogenes entering the gastrointestinal tract via L. monocytogenes-contaminated food, invading the intestinal mucosa, and entering the bloodstream via the intestinal barrier, causing bacteremia. When the body’s immunity is compromised, the bacterium releases hemolysin and Listerine, damaging the blood–brain barrier (BBB) and causing intracranial infection.12,14,15,20,21 In our study, all patients had a fever, three had nausea and vomiting during the disease. Blood tests revealed changes in infection index, including leukocytosis, monocyte percentage, CRP and IL-6 increase. Moreover, the blood culture of the fifth and eighth patients was positive at an early stage of the disease, indicating that these patients had bacteremia before the onset of neurological symptoms.
Like other forms of bacterial infections in the CNS, the most common signs and symptoms of CNS listeriosis are headache, fever, neck stiffness and disturbance of consciousness.7,22 However, some patients can present seizures and focal neurological deficits, such as cranial nerve palsies.23 Previous studies have demonstrated that only 41–51% of the patients presented the classic triad of fever, neck stiffness and altered mental status,22 which consistent with our research, 50% of the patients displayed a typical triad in this study. The examination of CSF in CNS listeriosis lacks specificity compared to the other bacterial infection of CNS, presenting intracranial hypertension, elevation leukocyte count and protein concentration, and decreased glucose levels. However, in our study, case one exhibited a normal CSF cell count, possibly due to the absence of leptomeningitis. The positive rate of CSF culture is approximately 85% before antibiotic treatment, while this ratio is significantly reduced when treated with antibiotics before lumbar puncture.22 In this study, the rate was only 28.57%.
Magnetic resonance imaging (MRI) presents the best soft tissue contrast detection and diagnostic sensitivity, and the acquisition of MRI sequences allows the distinctive visualization of brain anatomy with different contrast of structures.24 MRI may be useful in the diagnosis of CNS infections. Single or multiple nodular lesions with ring contrast enhancement with central cavities homogeneously hyperintense on diffusion-weighted MRI (DW-MRI) favor the diagnosis of infections.25,26 A prospective study of CNS listeriosis discovered that 83% of the cases have abnormal MRI findings.27 Leptomeningitis is the most common manifestation, followed by meningoencephalitis, while rhombencephalitis and brain abscess are rare. Previous studies have shown that brain abscess accounts for 1–5% of listeria infection.28,29 In our study, leptomeningitis, meningoencephalitis, and brain abscess all account for 25%, while brain MRI revealed no specific changes in the other two patients. In addition, CNS listeriosis appears more likely than other types of bacterial infection to involve the brainstem, as five cases exhibited consciousness disturbance, and two cases exhibited cranial nerve palsies, indicating brainstem involvement.
Diagnosing CNS listeriosis promptly is challenging due to the atypical clinical manifestations, laboratory examination, or MRI findings.9,27 L. monocytogenes isolation from blood or CSF combined with a compatible clinical manifestation is the gold standard for diagnosing neurolisteriosis.5,7,30 L. monocytogenes detection using the mNGS of CSF can also be used as evidence for diagnosing CNS listeriosis, and it appears to be more sensitive than traditional detection methods.31–33 The blood and CSF culture positive rates were 40% and 28.57%, respectively, while the mNGS positive rate was 100% in our cases. Simultaneously, blood tests present that CNS listeriosis is frequently associated with an elevated percentage of monocytes, while elevated neutrophils mainly characterize other bacterial infections, which is also an important basis for diagnosing neurolisteriosis. In our study, the percentage of monocytes increased is 60%. CNS listeriosis occasionally is misdiagnosed as tuberculous meningitis which usually requires treatment for more than 1 year;33 in our cases, five patients received empirical anti-tuberculosis treatment before identifying L. monocytogenes. When patients exhibit with prodrome characterized by gastrointestinal symptoms and a monocyte percentage increase, followed by manifestations of meningitis or meningoencephalitis, such as headache, fever, and altered consciousness, L. monocytogenes infection should be considered.17 Monitoring the proportion of monocytes in blood and improving mNGS of CSF contribute to early and rapid diagnosis.
There is currently no consensus concerning the regimen and duration of therapy for L. monocytogenes infection in the CNS.17,34 The European Society for Clinical Microbiology and Infectious Diseases (ESCMID) guideline recommends penicillin G, amoxicillin, or ampicillin as the standard treatment for listeria meningitis. Adding an aminoglycoside can be considered, and trimethoprim–sulfamethoxazole (SMZ-TMP), meropenem or linezolid would be effective alternatives.22 According to the literature, quinolones and rifampicin also have good results in L. monocytogenes infections as they can easily penetrate the BBB.35 Furthermore, ventriculoperitoneal drainage and lateral ventricular drainage are effective measures for patients with hydrocephalus.36 Moreover, L. monocytogenes infections of the CNS are associated with high mortality,28 with case fatality rates ranging from 20% to 60% despite the relatively sensitive antibiotic therapies.5,6,34,37,38 We have summarized three “2–3” principles for treating CNS listeriosis as it is difficult to select an adequate initial regimen due to the diagnosis delay.9 Our cases received empirical anti-tuberculosis treatment before definite diagnosis and adjusted to two to three sensitive antibiotics, including penicillin G, amoxicillin, meropenem, linezolid, SMZ-TMP, quinolones, and rifampicin. BBB permeability of these drugs can reach 20–30% at least in the case of meningeal inflammation. Among them, linezolid and quinolones can reach more than 60%.39 Meanwhile, case two and four underwent ventriculoperitoneal drainage and lateral ventricular drainage, respectively, due to hydrocephalus. The recommended treatment duration in the guideline is at least 21 days,22 and the time reported in previous cases varied from 10 days to 8 weeks due to the severity of the cases.35 In addition to patient one’s death from a brain hernia on the 15th day from the disease onset, the other patients in our study received sensitive antibiotic treatment for at least 2–3 months, with case five and six receiving treatment for 4 months due to their severe condition. Eventually, seven patients survived effective antibiotic treatment, while one died due to complications with a cerebral hernia at the early stage and delayed start of treatment in our report. Therefore, it is crucial to start appropriate antibiotics as soon as possible for a favorable outcome.
Limitation of the Study
There are some limitations to the present study. Firstly, the sample size we studied is small and has certain limitations. Secondly, this is a single-center retrospective study, potentially resulting in bias. Further multicenter prospective cohort study is needed to verify this conclusion.
Conclusion
In conclusion, L. monocytogenes infections should always be considered, especially in cases resistant to first-line antibiotic treatment. Monitoring the proportion of monocytes in blood and mNGS results of CSF can play a crucial role in diagnosing pathogens. Early and sufficient application of two to three sensitive antibiotics with a BBB permeability of 20–30% for at least 2–3 months can significantly improve the prognosis of CNS listeriosis.
Data Sharing Statement
The datasets are available from the corresponding author on reasonable request.
Ethical Approval
This study was conducted with the approval of the ethics committee of Chinese PLA General Hospital and followed the relevant provisions of the Helsinki Declaration of the World Medical Congress. The research only uses the previous medical record information and has removed the relevant personal information of the subject, which will not cause risks to the subject and will not have adverse effects on the rights and health of the subject, and the application for exemption of informed consent is approved at the same time. We will make every effort to protect the privacy and personal information of the subject’s personal medical data within the scope permitted by law.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Nyarko EB, Donnelly CW. Listeria monocytogenes: strain heterogeneity, methods, and challenges of subtyping. J Food Sci. 2015;80(12):M2868–M2878. doi:10.1111/1750-3841.13133
2. Matle I, Mbatha KR, Madoroba E. A review of Listeria monocytogenes from meat and meat products: epidemiology, virulence factors, antimicrobial resistance and diagnosis. Onderstepoort J Vet Res. 2020;87(1):e1–e20. doi:10.4102/ojvr.v87i1.1869
3. Cabal A, Pietzka A, Huhulescu S, Allerberger F, Ruppitsch W, Schmid D. Isolate-based surveillance of Listeria monocytogenes by whole genome sequencing in Austria. Front Microbiol. 2019;10:2282. doi:10.3389/fmicb.2019.02282
4. Thomas J, Govender N, McCarthy KM, et al. Outbreak of listeriosis in South Africa associated with processed meat. N Engl J Med. 2020;382(7):632–643. doi:10.1056/NEJMoa1907462
5. Scobie A, Kanagarajah S, Harris RJ, et al. Mortality risk factors for listeriosis – A 10 year review of non-pregnancy associated cases in England 2006–2015. J Infect. 2019;78(3):208–214. doi:10.1016/j.jinf.2018.11.007
6. de Noordhout CM, Devleesschauwer B, Angulo FJ, et al. The global burden of listeriosis: a systematic review and meta-analysis. Lancet Infect Dis. 2014;14(11):1073–1082. doi:10.1016/s1473-3099(14)70870-9
7. Zhao CW, Dai S, Wu Q. Pearls & Oy-sters: diagnosis and subtyping of listeria ventriculitis in an immunocompetent host. Neurology. 2022;99(3):123–126. doi:10.1212/WNL.0000000000200732
8. Li W, Bai L, Fu P, Han H, Liu J, Guo Y. The epidemiology of Listeria monocytogenes in China. Foodborne Pathog Dis. 2018;15(8):459–466. doi:10.1089/fpd.2017.2409
9. Choi MH, Park YJ, Kim M, et al. Increasing Incidence of Listeriosis and infection-associated clinical outcomes. Ann Lab Med. 2018;38(2):102–109. doi:10.3343/alm.2018.38.2.102
10. Zhang X, Feng P, Meng P, et al. Case report: severe listeria encephalitis with complicated or secondary autoimmune encephalitis and CNS demyelinating diseases. Front Public Health. 2022;10:848868. doi:10.3389/fpubh.2022.848868
11. Feng Y, Wu S, Varma JK, Klena JD, Angulo FJ, Ran L. Systematic review of human listeriosis in China, 1964–2010. Trop Med Int Health. 2013;18(10):1248–1256. doi:10.1111/tmi.12173
12. Pagliano P, Arslan F, Ascione T. Epidemiology and treatment of the commonest form of listeriosis: meningitis and bacteraemia. Infez Med. 2017;25(3):210–216.
13. Charlier C, Kermorvant-Duchemin E, Perrodeau E, et al. Neonatal listeriosis presentation and outcome: a prospective study of 189 cases. Clin Infect Dis. 2022;74(1):8–16. doi:10.1093/cid/ciab337
14. Radoshevich L, Cossart P. Listeria monocytogenes: towards a complete picture of its physiology and pathogenesis. Nat Rev Microbiol. 2017;16(1):32–46. doi:10.1038/nrmicro.2017.126
15. Disson O, Lecuit M. Targeting of the central nervous system by Listeria monocytogenes. Virulence. 2012;3(2):213–221. doi:10.4161/viru.19586
16. Frade HC, Pingili C, Nattanamai P. Multiple listeria abscesses in an immunocompetent patient. Cureus. 2020;12(1):e6642. doi:10.7759/cureus.6642
17. Mo T, Wu F, Dou X, Wang D, Xia H, Li X. A retrospective study of rare Listeria meningoencephalitis in immunocompetent children in China. Front Neurol. 2022;13:827145. doi:10.3389/fneur.2022.827145
18. Liu R, Wang J, Wang H, et al. Clinical retrospective analysis of 8 cases of central nervous system infection with Listeria monocytogenes. Chin J Infect Dis. 2022;40(2):98–102.
19. Vissing NH, Kristensen K, Monster MB, et al. Listeria meningitis in Danish children 2000–2017: a rare event even in a country with high rates of invasive listeriosis. Pediatr Infect Dis J. 2019;38(10):e274–e276. doi:10.1097/INF.0000000000002373
20. Banovic F, Schroten H, Schwerk C. Potential roles and functions of listerial virulence factors during brain entry. Toxins. 2020;12(5). doi:10.3390/toxins12050297
21. Shahid AD, Lu Y, Iqbal MA, et al. Listeria monocytogenes crosses blood brain barrier through Rho GTPases induced migration of macrophages and inflammatory interleukin expression. Microb Pathog. 2021;159:105143. doi:10.1016/j.micpath.2021.105143
22. van de Beek D, Cabellos C, Dzupova O, et al. ESCMID guideline: diagnosis and treatment of acute bacterial meningitis. Clin Microbiol Infect. 2016;22(Suppl 3):S37–62. doi:10.1016/j.cmi.2016.01.007
23. Yao M, Zhou J, Zhu Y, et al. Detection of Listeria monocytogenes in CSF from three patients with meningoencephalitis by next-generation sequencing. J Clin Neurol. 2016;12(4):446–451. doi:10.3988/jcn.2016.12.4.446
24. Alves AFF, Miranda JRA, Reis F, et al. Inflammatory lesions and brain tumors: is it possible to differentiate them based on texture features in magnetic resonance imaging? J Venom Anim Toxins Incl Trop Dis. 2020;26:e20200011. doi:10.1590/1678-9199-JVATITD-2020-0011
25. de Amorim JC, Torricelli AK, Frittoli RB, et al. Mimickers of neuropsychiatric manifestations in systemic lupus erythematosus. Best Pract Res Clin Rheumatol. 2018;32(5):623–639. doi:10.1016/j.berh.2019.01.020
26. Changa S-C, Laia P-H, Chena W-L, et al. Diffusion-weighted MRI features of brain abscess and cystic or necrotic brain tumors Comparison with conventional MRI. J Clin Imag. 2002;26:227–236. doi:10.1016/s0899-7071(02)00436-9
27. Charlier C, Poiree S, Delavaud C, et al. Imaging of human neurolisteriosis: a prospective study of 71 cases. Clin Infect Dis. 2018;67(9):1419–1426. doi:10.1093/cid/ciy449
28. Matthijs C, Sebastiaan GB, Gans JD, Spanjaard L, Gans JD. Community-acquired Listeria monocytogenes meningitis in adults. Clin Infect Dis. 2006;43(10):1233–1238. doi:10.1086/508462
29. Tiri B, Priante G, Saraca LM, Martella LA, Cappanera S, Francisci D. Listeria monocytogenes brain abscess: controversial issues for the treatment-two cases and literature review. Case Rep Infect Dis. 2018;2018:6549496. doi:10.1155/2018/6549496
30. Noll M, Kleta S, Al Dahouk S. Antibiotic susceptibility of 259 Listeria monocytogenes strains isolated from food, food-processing plants and human samples in Germany. J Infect Public Health. 2018;11(4):572–577. doi:10.1016/j.jiph.2017.12.007
31. Wang X, Guan H, Wei K, et al. Clincal data and next-generation sequencing results analysis of central nervous system infection with listeria. Chin J Neurol. 2018;51(6):451–455.
32. Zhang X, Wang R, Luo J, Xia D, Zhou C. Detection of meningoencephalitis caused by Listeria monocytogenes with ischemic stroke-like onset using metagenomics next-generation sequencing: a case report. Medicine. 2021;100(31):e26802. doi:10.1097/MD.0000000000026802
33. Lan ZW, Xiao MJ, Guan YL, Zhan YJ, Tang XQ. Detection of Listeria monocytogenes in a patient with meningoencephalitis using next-generation sequencing: a case report. BMC Infect Dis. 2020;20(1):721. doi:10.1186/s12879-020-05447-z
34. Fan Z, Xie J, Li Y, Wang H. Listeriosis in mainland China: a systematic review. Int J Infect Dis. 2019;81:17–24. doi:10.1016/j.ijid.2019.01.007
35. Pagliano P, Ascione T, Boccia G, De Caro F, Esposito S. Listeria monocytogenes meningitis in the elderly: epidemiological, clinical and therapeutic findings. Infez Med. 2016;24(2):105–111.
36. Liang JJ, He XY, Ye H. Rhombencephalitis caused by Listeria monocytogenes with hydrocephalus and intracranial hemorrhage: a case report and review of the literature. World J Clin Cases. 2019;7(4):538–547. doi:10.12998/wjcc.v7.i4.538
37. Ferna ́ndez Guerrero ML, Torres R, Mancebo B, et al. Antimicrobial treatment of invasive non-perinatal human listeriosis and the impact of the underlying disease on prognosis. Clin Microbiol Infect. 2012;18(7):690–695. doi:10.1111/j.1469-0691.2011.03616.x
38. Li C, Zeng H, Ding X, et al. Perinatal listeriosis patients treated at a maternity hospital in Beijing, China, from 2013–2018. BMC Infect Dis. 2020;20(1):601. doi:10.1186/s12879-020-05327-6
39. Nau R, Sorgel F, Eiffert H. Penetration of drugs through the blood-cerebrospinal fluid/blood-brain barrier for treatment of central nervous system infections. Clin Microbiol Rev. 2010;23(4):858–883. doi:10.1128/CMR.00007-10