Cancer and neoplasms
Algae: A robust living material against cancer
Introduction
Cancer is the second leading cause of death worldwide, and the incidence continues annually. National cancer statistics show that in 2016, the number of new cancers in China was 4.064 million, and the number of deaths from cancer was 2.414 million. According to the latest global cancer burden data released by the World Health Organization (WHO)’s International Agency for Research on Cancer (IARC), 19.29 million new cancer cases were diagnosed worldwide in 2020. Notably, 4.57 million cancer cases were diagnosed in China, accounting for 23.7% of the world.1 In light of the uncontrolled cell proliferation of cancer, cancer could also metastasize to other organs or tissues, posing a significant threat to cancer patients.2 Currently, basic and clinical research has been conducted extensively to develop new therapeutic strategies to extend life expectancy and reduce cancer mortality. Surgical resection, radiotherapy and chemotherapy remain the conventional treatment strategies for cancer at present. However, surgery, as one kind of local treatment for cancer, is only effective for cancer patients at the early stage. Radiation therapy, although effective, could inevitably pose significant unpleasant side effects on normal tissues. Chemotherapy could also systemically induce nonspecific systemic toxicity, such as gastrointestinal adverse reactions and immune-related diseases, which severely affect patients’ normal lives.3 In general, conventional cancer treatments such as surgical resection, radiation, and chemotherapy often lead to drug resistance and unpleasant side effects. However, in recent years, new anticancer therapies have emerged, including small-molecule targeted therapies, immunotherapy, antiangiogenic therapy, peptide or protein therapy, and gene therapy. Among these, natural products have gained attention because they tend to have fewer side effects than synthetic drugs. The exploration of anticancer drugs derived from natural products has become a hot topic in the field of cancer research both domestically and internationally. Algae, in particular, have shown significant potential in exhibiting anticancer activities.
China possesses abundant algae resources, presenting a significant potential for utilizing them to produce anticancer substances. According to the National Cancer Institute (NCI), approximately 3.5% of marine plants, including algae, exhibit anticancer or cytotoxic activity. Moreover, some algal species also possess antiviral and immunomodulatory effects, further adding to their therapeutic potential. Additionally, the chloroplasts present in algae can serve as a source of supplemental oxygen through photosynthesis, thereby aiding in the improvement of tumor hypoxia and inhibition of tumor growth.4 Photosensitizers in algae can be used in photodynamic therapy (PDT) to improve the efficiency of cancer treatment. Photosynthetic pigments such as chlorophyll, which are rich in algae, can be used as natural photosensitizers.5 Under specific light irradiation, photosensitizers can generate significant levels of reactive oxygen species (ROS), which possess the ability to induce cell death in tumor cells. It is worth noting that ROS can also play a role in supporting tumor metabolism, promoting angiogenesis, and modulating immune responses by influencing the tumor microenvironment and stromal cells.6 Algae can also serve as an efficient cancer drug delivery system, delivering chemotherapy or genetic drugs to cancer.5 Some algal extracts have been demonstrated to inhibit cell proliferation, metastasis, and tumor angiogenesis pathways while also promoting apoptosis, thereby exerting an anticancer effect. Algae are known to be rich in various natural ingredients, such as polysaccharides, proteins, terpenoids, sterols, polyphenols, cyclic polysulfide compounds, macrolides, and other bioactive substances. These components possess potent antiviral, anticancer, and antioxidant properties. Additionally, algae can be utilized in tumor therapy for diagnostic purposes through imaging techniques. To further enhance the biological activity and physical and chemical properties of algae, they can also be genetically engineered for targeted tumor treatment. These advancements hold great potential in expanding the application of algae in the field of cancer therapy.7 In the field of tumor therapy, algae can be utilized for tumor diagnosis through imaging techniques. They can be engineered to specifically target and identify tumor cells, aiding in the detection and localization of tumors. Furthermore, algae can be genetically engineered to enhance their biological activity and modify their physical and chemical properties, enabling them to effectively treat tumors. Algae have gained increasing attention as a significant biological resource for natural medicine. Being renewable in nature, algae offer an abundant source of medicinal substances. Thanks to advancements in modern biotechnology, the challenges associated with cultivating medicinal algae have been overcome, ensuring a sufficient supply of materials for the research and development of algae-derived anticancer substances. This breakthrough has opened up new avenues of exploration and possibilities for harnessing the potential of algae in the development of novel anticancer therapies.8 Furthermore, the improvement of modern separation and analysis techniques and the application of high-throughput screening techniques have led to the rapid development of algae as active anticancer substances. In view of the emerging field of algae, there have been several reviews on the application of algae in the field of food sources and liver diseases.9 However, to our knowledge, there has been no comprehensive and systematic review on the types, modification strategies, and therapeutic mechanisms of algae in cancer treatment. Therefore, we need a comprehensive overview of algae, as well as its application prospects and challenges, which will accelerate our understanding of advanced therapies in the design, engineering, and application of algae. We need to understand “how to classify algae”, “which engineering strategies could be used to modify algae”, and “the application of different algae and algae extracts in anticancer activity”. This will accelerate our understanding of advanced therapies for algal design, engineering and application while providing a comprehensive overview of these elements as well as application prospects and challenges.
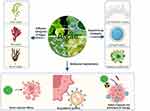
In this review, we intend to provide an overview of algae that can be used for anticancer purposes in recent years and their anticancer properties, strategies for engineering algae, and applications in tumor therapy. (Figure 1). First, we will provide an overview of the classification of algae and the engineering strategies employed in algae modification. This will lay the foundation for a preliminary understanding of the potential of algae in anticancer therapies. Next, we will present the latest research progress on the anticancer efficacy and mechanisms of algae. This will serve as a guide for researchers to identify new avenues for utilizing algae in anticancer approaches, enhancing their understanding of the field. Finally, we will discuss the prospects and challenges associated with the clinical translation of algae for cancer treatment. This will stimulate new ideas and perspectives on using algae as a therapeutic tool for diseases, contributing to advancements in the field. By combining these elements, we aim to provide a comprehensive understanding of the therapeutic applications of algae in cancer treatment. This will enable researchers to delve deeper into the potential benefits and challenges associated with algae-based therapies, fostering further innovation in the field.
|
Figure 1 Mechanistic illustration of algae against tumors. The illustration mainly consists of different categories of algae, engineering strategies, and anticancer mechanisms. Three engineering strategies, cell membrane coating, hydrogel loading and material modification, were adopted to realize sustained release, increased stability and enhanced biological properties for better anticancer activities. The anticancer mechanisms of algae include (1) direct cytotoxic effects, (2) drug delivery systems, and (3) relief of hypoxia and photodynamic therapy. Created with BioRender.com with enkaryotes and prokaryotes in the middle circle drawn by Adobe illulstrator. |
Classification of Algae
Algae, as photosynthetic organisms, can grow well in aquatic environments. Research on algae began in the 1950s, when researchers discovered that lipid-soluble extracts of Sphingomonas megasperma had antileukemic activity.10 This phenomenon has stimulated the interest of algae in their anticancer activity study. Many algal species with anticancer potential were identified from black seaweeds. The anticancer activity of 14 green algae, 23 brown algae, 23 cyanobacteria and 7 red algae was also found. Since the 1970s, components with anticancer activity have been isolated from a variety of algae.11 In the early 1980s, American and Japanese scientists began to systematically screen cyanobacteria and other algae for anticancer activity and discovered many new active compounds, such as scytophycin B extracted from cyanobacteria, which has entered the clinical stage.12 Many other bioactive substances extracted from algae have been clinically tested as anticancer drugs, and a significant portion of these substances from algae holds great potential for the development of anticancer natural drugs. For instance, Halomon isolated from Pinellia is also in the stage of preclinical pharmacological evaluation.13 Other anticancer substances from algae, such as cryptophycin, have been fully understood in terms of their mechanism of action and pharmacological effects. Lai et al synthesized cryptophycin-55/52 as an antibody‒drug conjugate (ADC). It was demonstrated that low concentrations of cryptophyll derivatives could inhibit mitotic proliferation without significantly altering the organization of spindle microtubules.14 The structure of malyngamide has been clarified, toxicological experiments have been completed, and in-depth studies have been conducted.15 Furthermore, algae polysaccharides, as plant polysaccharides, contain various biological activities with various functions, such as immunity, anticancer and antiradiation activity.16
Scientists mostly advocate the classification of algae into 11 phyla: cyanobacteria, red algae, cryptophytes, methanogens, golden algae, yellow algae, diatoms, brown algae, nudibranchs, green algae, and rotifers.17 There are many types of algae, and the first level of classification of algae is in accordance with the size of the algae, which can be classified as microalgae or macroalgae. Microscopic unicellular algae are called microalgae. Large multicellular algae visible to the naked eye are called macroalgae (seaweeds). The mega-algal family consists of Rhodophyta, Ochrophyta, and Chlorophyta and can be subsequently classified according to their hue and other characteristics. For example, algae can also be divided into three categories according to the color of their pigments: green algae, brown algae and red algae. Algal plants are mainly found in fresh or sea water and can also be divided into freshwater algae and marine algae. Among them, freshwater algae mainly include Chlamydomonas, Chlorella, Spirulina, macroalgae, and Rotifera. Marine microalgae are an important major source of novel beneficial genes and metabolites. Marine microalgae mainly include cyanobacteria, diatoms, green algae, etc.17,18 Algal extracts have a diversity of species and chemical compositions. Once they have been separated or extracted from algae, they may show a different spectrum of biological activities, such as antioxidant, antimicrobial, anticancer, antiallergy, antiviral and anticoagulant activities.19 Microalgal-derived phytochemicals are potentially biologically active in oxygen-producing photoreactivity and are often used to treat diseases exacerbated by hypoxia, such as ischemic heart disease, diabetic foot ulcers and cancer. In this review, algae are divided into prokaryotic algae and eukaryotic algae.20 Eukaryotic algae are a group of low-level autotrophic plants without root, stem or leaf differentiation and capable of photosynthesis. Morphology includes single cells, various groups, filaments, lobes, and tubular bodies. The size ranges from a few microns to several metermetres (kelp) and even a hundred metermetres (macroalgae). Generally, its structure is simple and has no obvious tissue differentiation. A few species have differentiation of the epidermis, cortex and pith, such as seaweed. Eukaryotic algae are usually divided into 10 phyla: Chlamydomonas (Chlorella), Chlorella, Nudibranchia, Diatoms, Chrysophyta, Methanophyta, Xanthophyta, Brown algae, and Red algae.21 Compared with eukaryotes, prokaryotes are relatively simple algae with a wide distribution, mostly in freshwater. Normally, they have single cells without a true nucleus, cell wall, populations or filopodia but no chloroplast membrane and no chloroplast formation, which are mainly cyanobacteria phyla (Cyanobacteria, Candida).22 In addition, there are a variety of photosynthetic pigments, such as chlorophyll a, phycocyanin, carotene, and carotenoids.
Engineering Strategies of Algae
Although algae possess several distinct properties, such as oxygen-producing capacity, photosensitive effects and biological effects, they often require further chemical or physical modification to improve their biological activities or physicochemical properties for therapeutic applications (Figures 1 and 2). For example, OU experiments were conducted using the polyaspartic acid (PASP) and Fe3+-mediated “Lego blocks” method with black phosphorus nanosheets (BPNSs) functionalized with active photosynthetic green algae (Chlorella vulgaris, Chl) for photocatalytic oxygenation for photosynthesis-enhanced synergistic photodynamic/chemodynamic/immunotherapy. Because most of the administered nanoparticles are captured by the liver and spleen mononuclear phagocyte systems, only a small amount of the agent can be deposited in the tumor, and therefore, the translational value of this approach is very limited.23 Thus, a series of engineering strategies have been applied to improve the biological functions of algae, including prolonging circulation or escaping clearance by the immune system in vivo, sustained release and stability enhancement by hydrogel incorporation, or material modification.
|
Figure 2 Engineered algae could ameliorate hypoxia to improve radiotherapy and PDT. (A) Illustrative description of the process and treatment of algae engineering. (B) Algal photograph. (C) SEM images of algae. (D) Engineered algae can effectively reach tumor sites and improve oxyhemoglobin after intravenous injection. (E) CD31 staining of cancer microvessels. (F) Engineered algae can increase oxygen, enhance radiation therapy, release chlorophyll, and induce PDT. (G) Confocal fluorescence images show elevated intracellular oxygen levels after treatment with engineered algae under hypoxic incubation conditions of the hypoxic probe. Reprinted from Qiao Y, Yang F, Xie T, et al. Engineered algae: A novel oxygen-generating system for effective treatment of hypoxic cancer. Science Advances. 2020;6(21):eaba5996. Creative Commons.24 |
Cellular membrane coating is a general strategy to prolong the circulation of nanoparticles or algae. For example, Wang et al proposed a tumor oxygenation strategy for self-oxygenating green algae, developed surface-engineered green algae, and constructed macrophage membrane-encapsulated Chlorella vulgaris (MCh 1) in this study, which can escape clearance by the immune system in vivo and achieve targeted enrichment of tumor tissues in response to inflammatory signals and continuously produce oxygen under laser transmission to improve the oxygen-depleted microenvironment in tumors, affecting the expression of drug efflux-related proteins and immunosuppression-related proteins and enhancing the anticancer efficacy of clinical first-line small molecule chemotherapeutic drugs and immunotherapeutic drugs, a potential adjuvant for clinical anticancer drugs that may benefit human health.25,26 Erythrocyte membrane engineering could also be applied to escape clearance by the immune system in vivo. Qiao et al modified Chlorella with erythrocyte membranes to effectively reduce the immunophagocytosis of Chlorella in immune cells and significantly decrease the clearance of macrophages, thus delivering Chlorella to tumor tissues more effectively. Using fluorescence imaging, the uptake of engineered Chlorella at the tumor site can be dynamically observed, and then the optimal time for radiotherapy can be selected; the dynamic changes in the blood oxygen content of engineered Chlorella in the tumor tissue can be observed by using photoacoustic imaging, which realizes real-time and dynamic monitoring of the tumor’s lack of oxygen.24
In addition to cell membrane engineering, hydrogel engineering is also a promising strategy for realizing the sustained release of algae and improving their stability. Hydrogels are polymer systems with a three-dimensional network structure containing large amounts of water formed by the simple reaction of one or more monomers. As polymeric materials, hydrogels swell with water and maintain large amounts of water in their structure but do not dissolve in water. To establish a local moist high-pressure oxygen atmosphere with dissolved oxygen delivery, Chen et al developed a patch wound dressing containing active elongated polycoccus. Apatch can enhance wound oxygenation, fibroblast proliferation, and angiogenesis, and it is nontoxic and immune inactive.27
Biomaterial modification can also be used for engineering algae to enhance their biological functions. Li et al obtained a biohybrid microalgae system by one-step biomimetic silicification of silica-coated Chlorella. Because of its high permeability and long retention effect, it was injected into mice and enriched in tumor sites, and its external silica coverage significantly reduced cytotoxicity and improved its bioactivity and tolerance in tumor areas. The increased oxygen concentration in tumor lesions and the release of chlorophyll enable cascade-enhanced radiotherapy and photodynamic therapy to significantly inhibit tumor growth.28
Application of Algae in Cancer Therapy
Application of Microalgae in Cancer Therapy
Microalgae are a common class of single-celled photosynthetic organisms, including prokaryotic algae (eg, cyanobacteria) and eukaryotic algae (eg, diatoms, green algae), which are widely found in ocean or freshwater lakes.17 In recent years, researchers have gradually recognized the great potential of microalgae as biomaterials for medical applications.29 Because microalgae are easy to obtain, easy to cultivate and have unique surface structures and abundant active substances, they have shown great advantages in imaging, drug delivery, oxygen-depleted cancer therapy, and wound healing.30 Microalgae strongly adsorb certain metal ions or micro- or nanoparticles, and the surface can be easily modified for targeted delivery of drugs. In addition to enabling drug delivery to treat cancer, microalgae can also photosynthesize and produce oxygen. Microalgae can photosynthesize with high photosynthetic efficiency and can continuously convert carbon dioxide to oxygen under light conditions. It can be used for local oxygenation, thus providing a source of reactive oxygen for highly oxygen-dependent treatments, for example, radiation therapy and photodynamic therapy, thereby improving the effectiveness of treatment. Microalgae-mediated photosynthesis generates oxygen in hypoxic cancer sites, significantly improving the hypoxic environment of cancers and the efficacy of radiation therapy.31 In addition, photosynthetic pigments such as chlorophyll rich in microalgae can also be used as natural photosensitizers, which not only further improve the efficacy of photodynamic therapy but also have fluorescence and photoacoustic imaging capabilities. A variety of components, such as lipids, proteins, polysaccharides, vitamins and antioxidants, are also present in microalgae. Increasing the activity of natural killer cells, activating the immune system, and inhibiting the growth of cancer cells are all ways microalgae enhance host defense. Thus, microalgae are considered to play an important role in cancer suppression.32
Application of Spirulina in Cancer Therapy
Spirulina is a class of prokaryotic organisms composed of single or multicellular filaments in loose or tight regular spiral shapes. The toxicity and side effects of radiotherapy and chemotherapy can be reduced, and immune function can be improved. Due to its hollow microhelical structure, Spirulina has been used to synthesize microswimmers for drug loading and cargo transport. Because of its structural and functional characteristics, Spirulina has some guidance for potential anticancer applications. In vitro and in vivo, magnetite nanostructured porous and hollow spiral microswimmers were fabricated from Spirulina for targeted delivery of therapeutic/imaging agents.33 Using Spirulina as a carrier, Zhong et al delivered adriamycin to targeted tissues.27 Zhou et al prepared an orally deliverable spirulina-amphotericin delivery system, SP@AMF, by loading Spirulina platensis with the radiation protection drug amphotericin through a simple dehydration-rehydration strategy.21 Spirulina-derived magnetic spiral microrobots (MoSBOTs) are being proposed by Victor as biotemplates for cancer treatment and biometrics.34 Zhong constructed SP@Curcumin, and two intestinal diseases can be treated with a drug delivery system, colon cancer and colitis, by using Spirulina platensis as a drug carrier for curcumin.25 In addition to drug delivery applications, Spirulina extracts also have certain anticancer effects. Michael et al found that carotene in spirulina kills tumor cells directly and prevents atherosclerosis. Liu et al also found that polysaccharides and phycocyanin in spirulina have significant inhibitory effects on cancer cells.
Application of Chlamydomonas in Cancer Therapy
Chlamydomonas is a single-celled green plant that is a common freshwater green algae. It is known as green yeast because it has characteristics similar to yeast, such as a simple growth cycle, fast growth, short generation time, monoclonal formation on a tablet, or liquid culture. Chlamydomonas can show active mobility and oxygen-producing photosynthetic activity, which suggests that Chlamydomonas can be used in the manufacture of microswimmers and artificial photosynthetic systems that can generate oxygen and thus improve cancer hypoxia and achieve cancer suppression. Chlamydomonas reinhardtii is a light-strategic green microalga that senses visible light and swims rapidly toward it. In C. reinhardtii, hydroxyproline-containing glycoproteins are dispersed on the negatively charged outermost layer, and positively charged nanomaterials (eg, polymers and magnetic materials) can adhere to the surface. Likewise, carbodiimide chemistry-mediated chemical conjugation allows for surface functionalization, and these advantages allow the microbe to be used as a microswimmer for anticancer applications and as a basis for photosynthesis.
Application of Chlorella in Cancer Therapy
Chlorella is an oxygen-producing photosynthetic green algae that can also display photosynthetic activity. Because of this feature, hypoxia-relieving cancer therapy can be delivered by actively producing O2 in cancer tissue after hybridization with functional substances. To help photosynthetic microorganisms (Chlorella) produce oxygen to enrich around the photosensitizer and access a continuous supply of oxygen, Wang developed a novel light-controlled sustainable PDT.35 To alleviate cancer hypoxia, Zhong et al used live photosynthetic microalgae (Chlorella vulgaris, C. vulgaris) as oxygenators.36 A live photosynthetic microalgae (Chlorella vulgaris, C. vulgaris) was used as an oxygenator to alleviate cancer hypoxia in Zhong et al. Chlorella AuNRs BSA-Gel, a BSA-PEG hydrogel reservoir system containing chlorella and gold nanorods, was developed by Li et al. This is considered to be an effective system for the treatment of hypoxic cancers.37 Ou et al used active photosynthetic green algae (Chlorella, Chl) functionalized with black phosphorus nanosheets (BPNSs) for photocatalytic oxygen release by using polyaspartic acid (PASP) and the Fe3+-mediated “Lego building block” method for photosynthesis-enhanced synergistic photodynamic/chemodynamic/immunotherapy.23 Zhou et al invented an innovative biologically produced nutrient material; a promising source of oxygen is the autotrophic light-triggered green feeder engine (ALGAE) formed using chlorella and alginate by calcium cross-linking.38 In addition to using photosynthetic activity to produce oxygen for anticancer activity, Chlorella contains rich bioactive substances that can also be used for anticancer activity. Chlorella polysaccharides and glycoproteins showed anticancer activity against ascites hepatocellular carcinoma AH44, AH41c, leukemia L-120, Ehrlich ascites carcinoma, and Meth A cancer. The combination of Chlorella glycoprotein and other anticancer drugs can enhance immunity, reduce the dosage of anticancer drugs, and improve the efficacy of treatment.
Application of Diatoms in Cancer Therapy
Diatoms are unicellular photosynthetic algae encased in siliceous outer walls. Siliceous cell walls are also called shells. Shell is a porous material in nature composed of silica, and mesoporous diatoms are mainly used in biosensing, photocatalysis and drug delivery. Natural silica is biocompatible, thermally stable, and chemically inert compared to synthetic silica.39 Since diatoms are highly porous, they provide multiple adsorption sites for chemotherapeutic agents and other nanoparticles, making them useful for cargo delivery.
Diatoms can also act directly as physisorbents, and surface chemical modifications of diatoms have been widely studied for cancer localization in animal models. Diatom cells as nanoparticles for chemotherapy drugs can kill cancer cells very precisely without harming normal cells. Australian scientists have modified diatoms so that antibodies and antigen-binding proteins are created on the surface of the shell. The shell surface produces binding proteins, and when the base-modified diatoms loaded with chemotherapy drugs are injected into patients, the antibodies only bind to molecules on cancer cells, delivering the drugs directly to the target cells.40 To deliver chemotherapeutic drugs to cancer cells, Delalat et al used diatom microalgae-derived nanoporous biosilica.40 Todd et al addressed the problem of in vivo delivery in complex environments by using diatoms as novel silica carriers.41 Diatoms are single-celled photosynthetic algae with silica shells encapsulated in porous 3D nanostructures called “truncates”. The shells of diatom truncates consist of biosilica self-assembled into complex porous shells with unique properties, such as customizable surface chemistry, thermal stability and high mechanical properties for chemical reactions. Diatom truncated bodies of richly available mineral deposits (diatomaceous earth or diatomaceous earth; DE) are used for the manufacture of diatom silica synthetic porous silica natural substitutes for biomedical, environmental, agricultural and energy applications. Shaheer et al summarized the applications of natural DE silica materials in biomedicine, focusing on drug delivery biosensing, tissue engineering and coagulation.42 Maher et al mechanically fragmented and transformed diatom silica structures into a novel mesoporous biodegradable silicon nanoparticle (SiNP), a drug carrier, by a magnesium thermal reduction process. The pH-dependent and sustained release of SiNPs loaded with adriamycin enhances the cytotoxicity of this drug in vitro. It has a significant increase in the cytotoxicity of cancer cells and considerable potential as a therapeutic diagnostic nanocarrier for chemotherapy.43,44
Application of Brown Algae in Cancer Therapy
In cold sea areas, brown algae are widely distributed, mainly including the genera Sargassum and Mercuria. The anticancer properties of brown algae are mainly achieved through their active substances. Brown algae contain fucoidan, which has anticancer properties and has been extensively researched, with the earliest research appearing in the 1980s.45 Numerous studies have shown that rockwort glycol can directly induce apoptosis and inhibit the cell cycle. It can also indirectly exert anticancer effects by activating natural killer cells and macrophages.46 Angiogenesis can also be inhibited, which reduces the growth and metastasis of cancer cells.47 Furthermore, rockweed polysaccharides have a wide range of biological activities. It has anti-inflammatory, antioxidant, anticoagulant, antithrombotic, antiviral, antiangiogenic, and anti-H. pylori properties.
Application of Cyanobacteria in Cancer Therapy
As gram-negative photoautotrophic prokaryotes without flagella, cyanobacteria are also known as blue‒green algae, containing chlorophyll a but not chloroplasts, which can photosynthesize and release oxygen to improve the hypoxic cancer environment.4 Using an electrostatic drop technique, Li prepared photosynthetic microcapsules (PMCs) by encapsulating cyanobacteria and UCNPs in alginate microcapsules (MCs). A durable oxygen supply was achieved. In addition to achieving an oxygen supply, cyanobacteria can be used as photosensitizers to enhance PDT for photodynamic therapy.48 Shih’s team constructed a photosensitizer dihydroporphyrin (ce6) hybridized with a cyanobacterium (Polypodium elongatum) for photosynthesis-enhanced PDT treatment of cancers under light excitation.23 To develop efficient strategies that can continuously improve the oxygen environment within cancers. To display good biocompatibility, Two-dimensional phosphorus nanosheets (BPNSs) was modified by photosynthetic cyanobacteria, thus constructing a novel bioreactor, Cyan@BPNSs.49,50 In this study, we provide a new strategy for the development of efficient and highly biocompatible photodynamic therapy platforms and further expand the application of microbial nanomedicines by hybridizing microorganisms with inorganic nanophotosensitizers.50
Application of Algae Extracts in Cancer Therapy
As a result of their large number of molecules, algae extracts have a high value, such as carbohydrates, proteins, peptides, lipids (including oils and polyunsaturated fatty acids, PUFAs), minerals, iodine, phenols (polyphenols, tocopherols), alkaloids, terpenes and pigments (eg, chlorophylls, carotenoids and phycocyanins). A compound’s biological activity may vary once it has been properly isolated or extracted from algae; these include antioxidants, antimicrobials, anticancer agents, antiallergic agents, antivirals, and anticoagulants.51,52 Natural extracts are superior to chemically synthesized drugs because they have high biological activity, wide sources, low resistance to drugs, and low side effects.
They can be used as novel anticancer drugs or as adjuvants in combination with anticancer drugs, such as algal polysaccharide complexes, mainly by enhancing immune system activity.2 Table 1 shows some anticancer mechanisms of algae extracts. Algae extracts have shown promising potential in fighting tumors through various mechanisms. These include inducing cell cycle block, triggering apoptosis (programmed cell death), inhibiting angiogenesis (the formation of new blood vessels), suppressing metastatic signaling pathways, regulating proteins involved in the cell cycle machinery, causing pathological alterations and DNA breaks, and inhibiting cellular activity (Figure 3).
|
Figure 3 Observations of the anticancer mechanism of algal extracts and their efficacy after treatment. (A) Molecular mechanism of the anticancer activity of algal extracts. Reprinted from Lin Y, Qi X, Liu H, et al. The anticancer effects of fucoidan: a review of both in vivo and in vitro investigations. Cancer Cell Int. 2020;20:154. Creative Commons.2 (B) Detection of apoptosis using live-dead cell cytostaining and flow cytometry. (a, AuNCs/HA upon NIR irradiation; b, DOX/AuNCs/HA; c, DOX/AuNCs/HA upon NIR irradiation; d, Reproduced with permission; doxorubicin (DOX); Gold nanorods (AuNRs); hyaluronic acid (HA)). Reprinted from Sun R, Chen H, Sutrisno L, et al. Nanomaterials and their composite scaffolds for photothermal therapy and tissue engineering applications. Sci Technol Adv Mater. 2021;22(1):404–428. Creative Commons.56 (C) Representative images of tumor immunohistochemical staining. (near-infra-red (NIR), photosynthesis microcapsule (PMC)). Reprinted from Wang W, Zheng H, Jiang J, et al. Engineering micro oxygen factories to slow tumor progression via hyperoxic microenvironments. Nature communications. 2022;13(1):4495. Creative Commons.48 |
|
Table 1 Anticancer Mechanism of Algae Extracts |
Microalgae have valuable medicinal properties. Among them, the growth of algal-derived compounds has received great attention in the pharmaceutical industry, and many cellular and molecular studies have shown that algal-derived compounds have naturally effective anticancer activity.41 Insights into novel cancer treatments may be improved by accumulating data on algae-derived anticancer resources.8
The carotenoid laminaria, found in microalgae, diatoms, and brown seaweed, has a variety of biological functions, such as anti-inflammatory, antioxidant, antidiabetic, hepatoprotective and anticancer activities, and it shows powerful anticancer properties by inhibiting cancer cell growth, stimulating oncogenes and blocking the cell cycle.57 In a variety of cancer cells, leptin induces apoptosis and cell cycle arrest, including human colon cancer (HCT116), human hepatocellular carcinoma (HepG2), human prostate cancer (LncaP and DU145), human promyelocytic leukemia (HL-60) and cervical cancer (HeLa) cells. Furthermore, it reduced Bcl-2 protein expression in antiapoptotic cells in prostate cancer and human promyelocytic leukemia, thus promoting apoptosis of cancer cells and exerting anticancer effects.51
Fucoidan from brown algae is used as an adjuvant for new anticancer drugs or in combination with anticancer drugs due to its high biological activity, wide availability, low drug resistance and low side effects. In terms of biological properties, it has anti-inflammatory, antiviral, antiangiogenic, immunomodulatory, anticoagulant and anti-adhesive effects.58 The anticancer activity of rockweed etiolated glycans is achieved through their ability to interfere with cancer initiation, development and progression through various mechanisms, including the proliferation and differentiation of cells, apoptosis, angiogenesis and metastasis.53 There are four main anticancer mechanisms of rockweed etiolated glycan. First, by inhibiting normal mitosis and regulating the cancer cell cycle, rockweed etiolated glycan can inhibit cancer cell proliferation. In a study by Alekseyenko et al, rockweed was injected into C57BL/6 mice transplanted with lung cancer; as a result, Rockweed et al demonstrated that it inhibited cancer cell metastasis and growth. Second, through related pathways, rockweed etan can activate the apoptotic signal of cancer cells and induce apoptosis, thus producing anticancer effects.59 Kim et al cocultured HT-29 and HCT 116 human colon cancer cells with rockweed etiolated glycans extracted from Morinda citrifolia. The results showed that rockweed etiolated glycan induced apoptosis in HT-29 and HVT 116 cells through cystatin-8 and −9-dependent pathways.54 Third, etiolated glycan in rock algae can inhibit the formation of VEGF (angiogenic endothelial factor), thus inhibiting angiogenesis, cutting off the supply of nutrients and oxygen to cancers, reducing the size of cancers, and blocking the spread and metastasis of cancer cells. By reducing the size of cancers and blocking their spread and metastasis. A study by Huang et al showed significantly lower levels of VEGF in the serum and lung tissue of mice implanted with lung cancer cells after receiving rockulan, and potent therapeutic effects could also be observed in mice transplanted with B16 melanoma, suggesting that the anticancer activity of rockweed etiolated glycan is related to its antiangiogenic effect.60 Fourth, rockweed glycan can also activate the body’s immune system and kill cancer cells by enhancing natural killer cells and T cells.48 In a study by Farzaneh et al, fucoidan sulfate was given to mice transplanted with acute promyelocytic leukemia cells, and rockweed etiolated glycans were found to enhance cell killing.61 Rockweed et al also showed that etiolated glycan inhibits the cell cycle and induces apoptosis in cancer cells, resulting in anticancer activity. Rockweed polysaccharide significantly inhibited growth and induced apoptosis in human hepatocellular carcinoma SMMC-7721 cells. Histological analysis of apoptosis in SMMC-7721 cells from hepatocellular carcinoma tissue was induced by rockweed etiolated glycan via the ROS-mediated mitochondrial pathway.62 Jang et al investigated the effect of rockweed etiolated glycan in human hepatocellular carcinoma cells and found that it significantly upregulated microRNA-29b (miR-29b). MiR-29b was dose-dependently induced by inhibition of its downstream target DNA methyltransferase 3B (DNMT3B). The protein levels of DNMT3B-suppressed messenger RNA and cancer metastasis suppressor gene 1 (MTSS1) were significantly increased by treatment with rocket algae etiolated glycans.63 In addition, rockulose downregulated transforming growth factor (TGF) receptors and SMAD signaling in hepatocellular carcinoma cells.63 The effects of these compounds inhibited the degradation of extracellular matrix and reduced HCC cell invasion. In BEL-7402 and LM3 cell lines treated with rockulose, p38MAPK/ERK pathway inhibition of cell proliferation was observed.64 Rockweed etiolated glycan inhibits PI3K activation, leading to ERK inhibition and MAPK activation. According to the results, there was a reduction in the ratio of Bcl-2 to Bax, leading to mitochondrial dysfunction, followed by increased caspase release, leading to apoptosis.65
Carotenoids are well-known antioxidants with anticancer activity. There are many types of carotenoids derived from microalgae, such as astaxanthin, which have anticancer properties. Human gastric cancer cell lines KATO-III and SNU-1 were found to be inhibited by astaxanthin in a dose-dependent manner. During the G0/G1 phase, the cell cycle was blocked.66 In addition, by regulating extracellular signal-regulated kinases (ERK) and cell cycle machinery proteins, it can increase the expression of p27 protein in gastric cell lines. Astaxanthin is a potent antioxidant that prevents oxygen-mediated genotoxicity and cytotoxicity, and hepatic xenotoxic metabolizing enzymes stimulate immunity against cancer.51
It is well known that blue‒green microalgae are neurotoxic with anticancer capabilities, and many active biological products can be isolated from them. For example, cryptophycin 1, derived from Nostoc sp. GSV 224, exhibits anticancer activity against solid cancers and human cancer cell lines. Cryptophycin-8, a semisynthetic form of cryptophycin, can show more potent antiproliferative effects in vivo.67 Another form of blue‒green microalgae is Cucarin-A isolated from Lyngbya majuscule, which can selectively inhibit various types of cancer cells.54 For example, Cucarin-A could inhibit the growth of kidney, colon and breast cell lines by inhibiting the activity of microtubulin polymerization. In addition, various benthic cyanobacterial strains may also show some anticancer effects on AML cells but not on noncancerous cells (eg, heart and liver cells).32
Calothrixins A and B from microalgae are phenanthridine alkaloids derived from Calothrixins sp., which have cytotoxic effects on human HeLa cells. Scytonemin is derived from Stigonema sp. and has shown antiproliferative effects on various forms of human fibroblasts and endothelial cells. Mitosis and the cell cycle are regulated by it.68 Another compound extracted from Symploca sp. is LU 103793. It has anticancer effects and interferes with the synthesis of microtubules.69 Largazole was also extracted from Symploca sp., and it showed strong anticancer effects, leading to the downregulation of type 1 histone deacetylase (HDAC).70 Phycocyanin is extracted from the microalga Spirulina and causes pathological changes and DNA fragmentation, upregulates Fas and ICAM expression, downregulates Bcl2 expression, and activates cystatin proteases in HeLa and MCF7 cell lines. An ethanolic extract of Aphanizomenon flos-aquae showed inhibitory effects on AML cells at the G0-G1 stage, as well as potent anticancer effects against MV-4-11 and HL-60 cells.32
However, obtaining biologically active substances is still difficult, and the small number of active compounds in the extracts, the failure to find adequate isolation and purification procedures, the possible toxicity of the compounds, the sustainability of the compounds, and the speed of their production are still problems that need to be solved.
Anticancer Mechanisms of Algae
Algae, as a biological material, can exert potent anticancer activity by a variety of mechanisms. In the following section, the anticancer mechanisms of algae will be categorized in terms of direct cytotoxic effects, drug delivery systems, photodynamic therapy and alleviation of hypoxia, and cancer imaging. (Figure 4).
|
Figure 4 Ideas and possible mechanisms of algae in tumor therapy. (A) Algae-based composite nanoparticles loaded with photodynamic chemotherapeutic immunomodulation to enhance tumor oxygenation and stimulate anticancer immune responses for cancer therapy. Aggregates specifically at the tumor site and penetrates flexibly into the tumor mass, thereby increasing the oxygenation state of the tumor. Adapted with permission from American Chemical Society: ACS Nano, Oxygen-delivering polyfluorocarbon nanovehicles improve tumor oxygenation and potentiate photodynamic-mediated anticancer immunity. Wang Z, Gong X, Li J, et al. Copyright© 2021 ACS Nano.71 (B) Possible mechanism of engineered algae-mediated combination therapy: Reduced HIF1 and VEGF protein levels promote apoptosis and necrosis. Reprinted from Qiao Y, Yang F, Xie T, et al. Engineered algae: A novel oxygen-generating system for effective treatment of hypoxic cancer. Science Advances. 2020;6(21):eaba5996. Creative Commons.24 (C) Engineered algae-mediated RT and PDT can effectively inhibit tumor growth after intravenous injection. Average cancer growth curve. (1, control; 2, laser alone; 3, RBCM-Algae alone; 4, x-ray irradiation (RT) alone; 5, RT + laser; 6, RBCM-Algae + laser; 7, RBCM-Algae + RT; and 8, RBCM-Algae + RT + laser. Student’s two-tailed t test, not significant P ≥ 0.05; *P < 0.05; ***P < 0.001; photodynamic therapy (PDT); red blood cell membrane (RBCM)). Reprinted from Qiao Y, Yang F, Xie T, et al. Engineered algae: A novel oxygen-generating system for effective treatment of hypoxic cancer. Science Advances. 2020;6(21):eaba5996. Creative Commons.24 (D) Kaplan‒Meier curve showing the survival rate of the indicated rabbits at 140 days. (near-infra-red (NIR), photosynthesis microcapsule (PMC), the data are presented as *p < 0.05). Nat Commun. 2022 Aug 2;13(1):4495. Open Access.48 (E) A regimen in which engineered algae-mediated RT and PDT can effectively inhibit tumor growth after intravenous injection. Reprinted from Qiao Y, Yang F, Xie T, et al. Engineered algae: A novel oxygen-generating system for effective treatment of hypoxic cancer. Science Advances. 2020;6(21):eaba5996. Creative Commons.24 (F) Effect of algae in a hyperoxic microenvironment on cancer. Representative axial CT images of tumor growth. (near-infra-red (NIR), photosynthesis microcapsule (PMC)). Reprinted from Wang W, Zheng H, Jiang J, et al. Engineering micro oxygen factories to slow tumor progression via hyperoxic microenvironments. Nature communications. 2022;13(1):4495. Creative Commons.48 |
Table 2 shows some examples of the anticancer mechanisms of algae.
|
Table 2 Anticancer Mechanisms of Algae |
Direct Cytotoxic Effects
The process of programmed cell death is known as apoptosis and refers to a physiological process in which normal cells are regulated by multiple genes under physiological conditions.47 Numerous genes have been reported to promote or inhibit apoptosis. Two types of genes play a regulatory role in the apoptotic process: cell death genes (Ced), such as the p53, Fas and cmyc oncogenes, and apoptosis suppressor genes, such as the bcl-2 gene family. Anthocyanins induce apoptosis in cancer cells mainly by regulating the expression levels of pro-apoptotic and anti-apoptotic genes26,72 Seaweed possesses biologically active compounds that promote apoptosis of cancer cells to make cancer cells more sensitive to chemotherapy drugs. A growing body of evidence has increasingly confirmed that compounds derived from algae can act as anticancer agents in several ways. For example, they inhibit cancer cell reproduction, invasion and metastasis and induce cell death through endogenous or exogenous apoptotic pathways, ultimately by the caspase pathway (cystatin protease).73
CD59 is a complement regulatory protein that is closely related to trauma, immune disorders and carcinogenesis and can protect blood cells while inhibiting the formation of the membrane attack complex (MAC), further promoting the immune escape of cancer cells.82 Phycocyanin exerts a dose-dependent anticancer effect on HeLa cells containing the CD59 gene. Some findings revealed that phycocyanin, as a mitogen, binds to the mitogen receptors on the surface of cancer cells, cross-linking the receptors with protein kinase activation, promoting intracellular CD59 protein transcriptional expression, inducing activation of the death structure domain and promoting cancer cell apoptosis.53
Drug Delivery Systems
Targeted drug delivery is the directed delivery or action of drug molecules to target tissues or cells. The common disadvantages of current drug delivery systems include poor drug solubility, short effective action time, and low bioavailability. Good biocompatibility makes microalgae an attractive drug delivery system, low cost, large surface area and active surface, and phototropism. Shchelik et al developed a novel microalgae-based drug delivery system that could release vancomycin under light conditions and synergize with UV therapy, and infections of the skin can be treated with this medication.74 Weibel et al designed a drug-carrying microswimmer using the abovementioned properties of microalgae. The microalgae are guided to the target site by their inherent phototropism, irradiated with ultraviolet light and controlled drug release.75 Akolpoglu et al used chitosan-coated iron oxide nanoparticles to modify the surface of Chlamydomonas reinhardtii and construct a drug-loaded microphoresis apparatus. Improving the adhesion ability of nanoparticles while maintaining their inherent phototropism and migration.75
Chlamydomonas (single-celled and photosynthetic microalgae) shows active mobility and oxygen-producing photosynthetic activity, and C. reinhardtii is a light-strategic green microalga that senses visible light and swims rapidly toward it. Experimentally fabricated biohybrid microswimmers have shown excellent on-demand drug delivery capabilities to SK-BR-3 cancer cells and can be used for effective delivery of chemotherapeutic cargo.30 Sitti’s team developed a biohybrid microalgal microswimming system. Photolyzed iron oxide nanoparticles were also attached to chitosan in a biohybrid microphoresis apparatus to change the DOX loading, which exhibited excellent targeted drug delivery to cancer cells and were able to achieve on-demand drug release. This confirms that the developed microalgae-based microswimmers can be used for the delivery of active chemotherapeutic agents that can be used. Since C. reinhardtii is a phototropic green microalgae, it is capable of sensing visible light and swimming toward it at high speeds.76 Surface chemistry could be used to attach it to polystyrene (PS) beads, perform photomigration and microscale loading, and release the attached loading drugs by photochemistry.30
With their inherent high porosity, diatoms can adsorb many nanoparticles and chemotherapeutic drugs. In cargo delivery systems, chitosan can be widely used. Chemical modifications can be made to the surface of diatoms. Diatoms can be modified with dopamine and iron oxide nanoparticles to provide fine-tuned and magnetically guided drug delivery. Using diatom shells as a drug delivery system, without the use of other chemicals, layers of chitosan can be created to act as pH-responsive nanovalves to regulate drug doses in cancer. Magnetized diatoms can be loaded with large quantities of drugs and controlled by magnetic fields for targeted drug transport and slow release. Yasa et al coincubated polyelectrolyte-functionalized 1 μm diameter magnetic polystyrene particles with Chlamydomonas reinhardtii, and the two were combined by noncovalent interactions to form a biohybrid microphoresis apparatus, which enables targeted delivery of magnetic particles under the action of a magnetic field.83 Losic et al attached dopamine-modified ferric tetroxide magnetic nanoparticles to diatoms by a simple one-step electrostatic self-assembly method and verified the drug delivery effect with indomethacin and gentamicin.77 Using nanoporous biosilica derived from diatom microalgae, Bahman et al delivered chemotherapeutic drugs to cancer cells.33 Todd et al addressed the problem of in vivo delivery in complex environments by using diatoms as novel silica carriers. Diatoms as carriers can be loaded with much higher payloads than ordinary silica nanoparticles.41 Shaheer et al summarized the applications of natural DE silica materials in biomedicine, focusing on drug delivery biosensing, tissue engineering, and coagulation.42 Maher et al mechanically fragmented and transformed diatom silica structures into a novel mesoporous biodegradable silicon nanoparticle (SiNP), a drug carrier, by a magnesium thermal reduction process. SiNPs loaded with adriamycin released the drug in vitro in a pH-dependent manner and showed enhanced cytotoxicity against cancer cells. In addition to enhanced cytotoxicity against cancer cells, it has considerable potential as a therapeutic diagnostic nanocarrier for chemotherapy.43,44
Spirulina’s cell membrane is rich in channels and pores. In addition to adsorbing positively charged DOX molecules to negatively charged membranes, it can also encapsulate cyanobacteria (molecules entering the periplasm of the cell), and the drug loading efficiency was 85%, resulting in a high drug loading efficiency.30 Zhong et al used Spirulina platensis (a type of cyanobacteria) as a carrier to achieve targeted delivery of adriamycin. A noncovalent electrostatic interaction between positively charged adriamycin and negatively charged Spirulina platensis surface enabled drug loading. The water channels and pores on the surface of Spirulina platensis allowed the entry of small molecules, thus further increasing the drug loading rate.78 For therapeutic and biometric applications, de la Asunción‐Nadal et al proposed the use of MoS2-based magnetic spiral microrobots (MoSBOTs) of the cyanobacterium Spirulina. They are cytocompatible and can harvest MoS2 photothermal activity in near-infrared radiation. MoSBOT’s light absorption properties enable targeted photothermal ablation of cancer cells and real-time biometrics. Zhong et al used Spirulina platensis (Spirulina obtusa) as a drug carrier for curcumin to develop a drug delivery system. Two intestinal diseases, colon cancer and colitis, can be treated with SP@curcumin.25,34
Relief of Hypoxia and Photodynamic Therapy
Tumor cells proliferate rapidly, and abnormal blood vessels form. There is widespread hypoxia in rapidly growing solid tumors. Radiation therapy and photodynamic therapy are adversely affected by tumor hypoxia. Therefore, improving the hypoxic microenvironment of tumors would greatly enhance the effectiveness of radiotherapy/photodynamic cancer therapy. Previous studies have attempted to use nanoparticles to generate oxygen or administer oxygen in situ to tumors with the aim of improving the therapeutic effect. (Figure 5). However, in the liver and spleen, mononuclear phagocytes capture most nanoparticles. Only approximately 2% can successfully reach the tumor site, resulting in high-dose delivery and systemic toxicity that limit their application. In recent years, many studies have utilized the photosynthetic in situ oxygen production of microalgae to improve tumor hypoxia and enhance tumor therapeutic effects.
|
Figure 5 Algae combined with photodynamic anticancer experiments and cell validation experiments. (A) Schematic diagram of algae-based PDT to enhance anticancer effects. Reprinted from Wang H, Liu H, Guo Y, et al. Photosynthetic microorganisms coupled photodynamic therapy for enhanced antitumor immune effect. Bioact Mater. 2022;12:97–106. Creative Commons.84 (B) Photograph of the tumor at the end point. (PBS, PBS plus laser irradiation (PBS+L), nanoparticles (PDG), PBS plus laser irradiation (PDG+L), gemcitabine (Gem), sensitive prodrug of chemo-immunomodulatory gemcitabine (PF11DG), the treatment of PF11DG plus laser irradiation (PF11DG+L). Adapted with permission from American Chemical Society: ACS Nano, Oxygen-delivering polyfluorocarbon nanovehicles improve tumor oxygenation and potentiate photodynamic-mediated anticancer immunity. Wang Z, Gong X, Li J, et al. Copyright© 2021 ACS Nano.71 (C) Scratch experiments were used to demonstrate the cellular effects of infrared light irradiation signaling pathways. (near-infra-red (NIR), photosynthesis microcapsule (PMC). Reprinted from Wang W, Zheng H, Jiang J, et al. Engineering micro oxygen factories to slow tumor progression via hyperoxic microenvironments. Nature communications. 2022;13(1):4495. Creative Commons.48 (D) To demonstrate in vivo tumor targeting, permeability, etc. In vivo tumor accumulation of photodynamic agents in mice under an in vivo imaging system. Adapted with permission from American Chemical Society: ACS Nano, Oxygen-delivering polyfluorocarbon nanovehicles improve tumor oxygenation and potentiate photodynamic-mediated anticancer immunity. Reprinted from Wang W, Zheng H, Jiang J, et al. Engineering micro oxygen factories to slow tumor progression via hyperoxic microenvironments. Nature communications. 2022;13(1):4495. Creative Commons.71 (E) Staining of new daughter cells, confocal visualization. (near-infra-red (NIR), photosynthesis microcapsule (PMC)). Reprinted from Wang W, Zheng H, Jiang J, et al. Engineering micro oxygen factories to slow tumor progression via hyperoxic microenvironments. Nature communications. 2022;13(1):4495. Creative Commons.48 (F) Cell proliferation assessment Photosynthetic microcapsules prepared from algae are less cytotoxic and can inhibit cancer cells in the presence of infrared light. (near-infra-red (NIR), photosynthesis microcapsule (PMC), the data are presented as mean ± SD. ***p < 0.001 compared to control cells according to two-tailed Student’s t-test). Reprinted from Wang W, Zheng H, Jiang J, et al. Engineering micro oxygen factories to slow tumor progression via hyperoxic microenvironments. Nature communications. 2022;13(1):4495. Creative Commons.48 |
|
Figure 6 In addition to anticancer activity, algae exhibit hemostatic, antibacterial and prorepair-oriented anticancer effects postoperatively, and other issues need to be studied in depth. (A) Schematic representation of the main mechanism of action of antimicrobial compounds derived from macroalgae. Reprinted from Silva A, Silva SA, Carpena M, et al. Macroalgae as a Source of Valuable Antimicrobial Compounds: Extraction and Applications. Antibiotics (Basel). 2020;9(10). Creative Commons.88 (B) Schematic diagram of the combination of algae and hydrogel for wound hemostasis. Reprinted from Zou CY, Lei XX, Hu JJ, et al. Multicrosslinking hydrogels with robust bioadhesion and pro-coagulant activity for first-aid hemostasis and infected wound healing. Bioact Mater. 2022;16:388–402. Creative Commons.89 (C) Schematic diagram of algae promoting chronic wound repair. Reprinted from Chen H, Cheng Y, Tian J, et al. Dissolved oxygen from microalgae-gel patch promotes chronic wound healing in diabetes. Science advances. 2020;6(20):eaba4311. Creative Commons.55 (D) The shallower wounds are covered by algae, thus maintaining a good moist environment and promoting wound repair and healing. Reprinted from Chen H, Cheng Y, Tian J, et al. Dissolved oxygen from microalgae-gel patch promotes chronic wound healing in diabetes. Science advances. 2020;6(20):eaba4311. Creative Commons.55 |
The chlorophyll in Spirulina acts as a photosensitizer to kill tumor cells by generating reactive oxygen species under laser irradiation, thus enabling photodynamic therapy. The efficient synergy of radiotherapy/photodynamic therapy can greatly enhance its ability to kill tumor cells.80 Under red light-induced photosynthesis, Qiao et al proposed an innovative method for producing O2 in mouse tumors in situ.39
Conditions, it can accumulate at the tumor site and produce oxygen to facilitate the tumor’s response to radiation. Additionally, it contains chlorophyll, a natural photosensitizer. When laser radiation is applied to the tumor site, it can accumulate and produce oxygen to facilitate the response to radiation.81 Lee et al developed a hydrogel system containing Chlorella and gold nanorods, which was injected in situ into the tumor sites of 4T1 mammary carcinoma mice and was able to increase the local oxygenated hemoglobin in the mice tumors by generating large amounts of oxygen through photosynthesis under 660 nm laser irradiation. Chlorella vulgaris was encapsulated in red blood cell membranes (RBCMs) by Zhou et al. An oxygen-producing biohybrid system could be successfully delivered to tumor tissues under red light-induced photosynthesis to achieve hypoxia relief and enhance radiation treatment. Subsequent release of chlorophyll from microalgae after laser irradiation can generate ROS, which further confers PDT, leading to further complete tumor ablation.68 In addition, the Algae@SiO2a natural photosensitizer chlorophyll is found in Chlorella vulgaris, which can be used in photodynamic therapy (PDT).79 Ou et al used active photosynthetic green algae (Chl) functionalized by black phosphorus nanosheets (BPNSs) to achieve synergistic enhancement of photodynamic/chemodynamic/immunotherapy on photosynthesis. A major benefit of Chl is that it has a positive effect on cancer tissue hypoxia, enhances immune cell infiltration, and stimulates the proliferation and maturation of immune cells. BPNSs are loaded on the surface of Chl cells and can be used for oxygen production and release, thereby increasing the efficiency of light conversion and offering great potential for photodynamic and immunosynergistic therapeutic clinical applications.23
Zhou et al invented ALGAE, a nutritious light-triggered green feed engine, where protein-nucleated Chlorella is cross-linked with alginate by calcium.38 It is a bio-oxygenation engine for the treatment of hypoxia-resistant cancer. In addition, the green oxygen-producing engine is biocompatible and biodegradable, which can generate large amounts of oxygen around tumor tissue when irradiated while enhancing PDT. It can also produce long-term repeated oxygen supplementation to reduce tumor hypoxia, downregulate the expression of HIF-1α and VEGF, and increase the expression of calreticulin (E-cadherin), thus inhibiting tumor cell metastasis and eradicating cancer.38 In Wang’s experiments, light was used to help Chlorella produce oxygen, leading to the development of a new light-controlled sustainable PDT, which can continuously provide oxygen to the photosensitizer, prevent local hypoxia caused by PDT and promote DC activation. The new strategy improves PDT in hypoxic tumors in a simple and effective manner. Boosting anticancer immunity may be an effective strategy against drug resistance in advanced cancer patients.35
Under 625 nm laser irradiation, phycocyanin (C-PC) induced cytotoxic stress via ROS, and apoptotic cell death phenomena such as cell shrinkage, cytoplasmic agglutination, nuclear division, and apoptotic vesicle formation occurred after laser treatment.4 Shi’s team constructed a photosensitizer dihydroporphyrin (ce6) hybridized with a cyanobacterium (Polyphyllum elongatum) for photosynthesis-enhanced PDT treatment of tumors under light excitation. Based on the similarity between the chlorophyll structure of cyanobacteria and the photosensitizer structure, this photosensitized bacterium can achieve photosynthesis and photosensitizer activation under a single light source excitation (660 nm) to cause efficient killing of tumor cells.4 To develop efficient strategies that can continuously improve the oxygen environment within tumors. Lin et al used inorganic two-dimensional black phosphorus nanosheets (BPNSs) to modify photosynthetic cyanobacteria with good biocompatibility, thus constructing a novel bioreactor, Cyan@BPNSs. Under laser irradiation, cyanobacteria can continuously generate oxygen by photosynthesis, and subsequently, BPNSs will photosensitively activate oxygen to generate singlet oxygen (1O2). It can accumulate in large numbers in tumor sites and show a strong killing effect on tumor cells.50
Application of Algae in Cancer Imaging
Imaging plays a very important role in cancer diagnosis and treatment, and imaging examinations are indispensable for both the initial diagnosis and review of cancer. For example, lung cancer patients usually undergo chest CT to determine the site of the lesion in the initial diagnosis and to compare whether the cancer has progressed in the review, including interstitial pneumonia, pulmonary fibrosis and other lung complications, which can be helped by CT examination.85 MRI imaging has better contrast between lesions and normal tissue, making it easier to detect small lesions.86 The image comparison before and after contrast injection can be used to observe the travel and distribution of blood vessels and assist in diagnosis. Ultrasound examination has the advantages of high safety, ease of use and low cost. Ultrasound is commonly used by lung cancer patients to detect whether there are distant metastases, such as liver, kidney, and lymph nodes around the abdominal artery.87
Microalgae can be applied to bioimaging due to their easy culture, good biocompatibility, complex and precise active surface, and rich chlorophyll content. Carbon quantum dots and multivacancy carbon made from microalgae by a one-step hydrothermal method have high cellular uptake, low toxicity and two-photon fluorescence properties, which have high translational potential for cellular imaging. Zhong et al used live photosynthetic microalgae (C. vulgaris) as an oxygenator to experimentally modify the surface of the photosynthetic microalgae and form a bionic system (CV@CaP) to regulate tumor hypoxia for image-guided collaborative treatment. Photosynthesis of CV@CaP can alleviate oxygen production by in situ tumor hypoxia and significantly improve the effect of RT.36
The bioengineered diatoms show IgG platforms that can be easily functionalized with appropriate functional materials. Todd et al used diatoms encapsulated with a dye molecule, iron oxide np, which combines the advantages of FL and MR imaging and can be used to identify cancers in animals.41
The chlorophyll in Chlorella is autofluorescent and is visible through in vivo fluorescence imaging systems. Li et al prepared Algae@SiO2 based on Chlorella vulgaris, and algae@SiO2 has UV‒vis absorption similar to that of algae. According to the UV‒vis spectrum and Algae@SiO2, 680 nm was observed as the maximum absorption peak. Using the photoacoustic effect, photoacoustic (PA) imaging can be performed. This can create a visual diagnosis by converting pulsed laser excitation into detectable ultrasound signals. It allows imaging deep into the tissue while maintaining high spatial resolution. Therefore, Algae @SiO2 can be used as an exogenous contrast agent for PA imaging.79
Cyanobacteria can photosynthesize and release oxygen. Spirulina is a subspecies of cyanobacteria. Using Spirulina as a driver, Zhou et al constructed a photosynthetic biohybrid microswimmer (pbn) by functionally transforming Fe3O4 nanoparticles into microalgae cells to enable in vivo cancer targeting and MR imaging. Spirulina can also synthesize the natural photosensitizer chlorophyll for photosynthesis, producing oxygen in situ to improve cancer hypoxia and enhance RT efficacy, enhancing PDT effects by producing cytotoxic ROS under laser irradiation.
According to the summary of algae in imaging, we found that algae are also very widely used in cancer imaging; they can localize cancer cells due to their own fluorescence properties, can produce oxygen to enhance the PDT effect, can be combined with other biological materials to localize cancers or for imaging guidance, and can also participate in radiotherapy as contrast agents. However, the number of algal species that we can use for cancer imaging is relatively small and needs to be further investigated.
Challenges and Opportunities
While algae show potential in fighting tumors, there are still challenges that need to be addressed. Limited survival of algae due to light conditions and incubation temperatures, as well as high production costs and imperfections in culture and extraction techniques, hinder the industrialization of algae. Additionally, the active substances found in algae may exhibit toxicity toward humans. The use of algae in drug delivery for anticancer purposes may face issues such as particle aggregation and reduced efficacy. Furthermore, there is a risk of infection, inflammation, and immune response associated with the use of algae with photodynamic systems for treating tissue hypoxia.
Despite these challenges, increasing concerns about climate change and sustainable development have led to the promotion of algae as a strategy for carbon neutrality, which may foster innovative techniques for algae industrialization. The oxygen-supplying capacity of algae can help combat multidrug resistance and immunosuppression caused by the tumor microenvironment. The active ingredients in algae can enhance the sensitivity of tumor cells to chemotherapeutic agents by altering cellular drug transport mechanisms. Algae’s biosafety and biodegradability reduce the risk of side effects, making them a promising microplatform for lung-targeted drug delivery and fluorescence imaging-guided chemotherapy for cancer lung metastases.
In addition to their potential in antitumor applications, algae have shown promise in hemostasis, antimicrobials, and tissue repair. Certain types of algae components can promote blood clotting and constrict blood vessels, aiding in wound healing and stopping bleeding. Algae also possess antioxidant properties and exhibit bactericidal activity against certain strains of bacteria. With their favorable hydrophilicity and biocompatibility, algae can effectively cover shallow wounds, maintaining a moist environment conducive to wound repair and healing (Figure 6).
Overall, while there are challenges and limitations, engineered algae hold promise in the integration of tumor therapy and tissue repair. Further research and optimization are needed to fully exploit their potential in these areas.
Conclusion and Outlook
The current review highlights the promising research on the use of algae for anticancer therapy, indicating a bright future in this field. Algae offer a new direction for the development of novel anticancer drugs due to their effectiveness and low side effects. Their easy availability and reproducibility make them abundant sources of anticancer substances, providing ample materials and possibilities for research.
Algae exhibit various anticancer mechanisms, such as improving the hypoxic tumor environment, enhancing photodynamic therapy, acting as carriers for drug delivery, serving as natural photosensitizers in tumor imaging, and impacting the cell lineage and cell cycle of tumors. These diverse mechanisms greatly enhance the anticancer efficacy of algae.
However, there are still challenges to overcome in the field of algae-based anticancer therapy. Many algae still rely on a single mechanism of action, and there is room for exploring their synergistic anticancer activity. The stability of algae and algal extracts needs further verification, and the engineering modifications of algae remain limited and require more research. Additionally, most anticancer studies of algae are restricted to small animal models, and further data are needed to support their clinical application. Moreover, when combined with biomaterials, the safety of these materials needs to be rigorously evaluated. The translation of algae from basic research to clinical application still has a long way to go.
With the increasing demand for sustainable development and environmental protection, the use of algae and research in this area will continue to be promoted. The active ingredients in algae, such as polysaccharides and polyphenols, possess a wide range of medicinal properties, including anti-inflammatory, antioxidant, anticancer, and immunomodulatory activities. They hold great potential as sources for drug development and therapy. Moreover, algae can find applications in biotechnology, including biosensors, biodyes, and biomaterials. As new diseases emerge, there will undoubtedly be increased research on marine medicines for better disease treatment.
In conclusion, the use of algae for anticancer therapy shows great potential, but there are still challenges to be addressed. Further research and development are necessary to fully exploit the benefits of algae in this field and translate them into clinical practice.
Funding
This work was supported by the National Natural Science Foundation of China [82072051, 81771964].
Disclosure
The authors report no conflicts of interest in this work.
References
1. Cao W, Chen HD, Yu YW, et al. Changing profiles of cancer burden worldwide and in China: a secondary analysis of the global cancer statistics 2020. Chin Med J. 2021;134(7):783–791. doi:10.1097/CM9.0000000000001474
2. Lin Y, Qi X, Liu H, et al. The anticancer effects of fucoidan: a review of both in vivo and in vitro investigations. Cancer Cell Int. 2020;20(1):154. doi:10.1186/s12935-020-01233-8
3. Yau SH. Immunotherapy for metastatic cancer patients: the current status, limitations, obstacles and future directions. Ann Palliat Med. 2020;9(2):121–125. doi:10.21037/apm.2020.02.23
4. Huo M, Wang L, Zhang L, et al. Photosynthetic tumor oxygenation by photosensitizer‐containing cyanobacteria for enhanced photodynamic therapy. Angew Chem Int Ed. 2020;59(5):1906–1913. doi:10.1002/anie.201912824
5. Ferdous UT, Yusof ZNB. Medicinal prospects of antioxidants from algal sources in cancer therapy. Front Pharmacol. 2021;12:593116. doi:10.3389/fphar.2021.593116
6. Cheung EC, Vousden KH. The role of ROS in tumor development and progression. Nat Rev Cancer. 2022;22(5):280–297. doi:10.1038/s41568-021-00435-0
7. Pereira L. Characterization of bioactive components in edible algae. Mar Drugs. 2020;18(1):65. doi:10.3390/md18010065
8. Alves C, Silva J, Pinteus S, et al. From marine origin to therapeutics: the anticancer potential of marine algae-derived compounds. Front Pharmacol. 2018;9:777. doi:10.3389/fphar.2018.00777
9. Li Y, Zhao L, Li XF. Hypoxia and the tumor microenvironment. Technol Cancer Res Treat. 2021;20:15330338211036304. doi:10.1177/15330338211036304
10. Ermakova S, Sokolova R, Kim SM, et al. Fucoidans from brown seaweeds Sargassum hornery, Eclonia cava, Costaria costata: structural characteristics and anticancer activity. Appl Biochem Biotechnol. 2011;164(6):841–850. doi:10.1007/s12010-011-9178-2
11. Liu Q, Meng Q. In vivo antitumor effect of polysaccharide from Sargassum confusum and the mechanisms. Acad J First Med Coll PLA. 2004;24(4):434–436.
12. Silva-Stenico ME, Kaneno R, Zambuzi FA, et al. Natural products from cyanobacteria with antimicrobial and antitumor activity. Curr Pharm Biotechnol. 2013;14(9):820–828. doi:10.2174/1389201014666131227114846
13. Kadam SU, Tiwari BK, O’Donnell CP. Application of novel extraction technologies for bioactives from marine algae. J Agric Food Chem. 2013;61(20):4667–4675. doi:10.1021/jf400819p
14. Lai Q, Wu M, Wang R, et al. Cryptophycin-55/52 based antibody-drug conjugates: synthesis, efficacy, and mode of action studies. Eur J Med Chem. 2020;199:112364
15. Moss NA, Leão T, Rankin MR, et al. Ketoreductase domain dysfunction expands chemodiversity: malyngamide biosynthesis in the cyanobacterium Okeania hirsuta. ACS Chem Biol. 2018;13(12):3385–3395. doi:10.1021/acschembio.8b00910
16. Sanjeewa KKA, Jeon YJ. Fucoidans as scientifically and commercially important algal polysaccharides. Mar Drugs. 2021;19(6):284. doi:10.3390/md19060284
17. Funk VA, Herendeen P, Knapp S. Taxonomy: naming algae, fungi, plants. Nature. 2017;546(7660):599. doi:10.1038/546599c
18. Qian P, Zhao Z, Liu H, et al. Multi-target deep learning for algal detection and classification. Annu Int Conf IEEE Eng Med Biol Soc. 2020;2020:1954–1957. doi:10.1109/EMBC44109.2020.9176204
19. Frazzini S, Scaglia E, Dell’Anno M, et al. Antioxidant and antimicrobial activity of algal and cyanobacterial extracts: an in vitro study. Antioxidants. 2022;11(5). doi:10.3390/antiox11050992.
20. Jaubert M, Bouly JP, Ribera d’Alcalà M, et al. Light sensing and responses in marine microalgae. Curr Opin Plant Biol. 2017;37:70–77. doi:10.1016/j.pbi.2017.03.005
21. Rockwell NC, Duanmu D, Martin SS, et al. Eukaryotic algal phytochromes span the visible spectrum. Proc Natl Acad Sci U S A. 2014;111(10):3871–3876. doi:10.1073/pnas.1401871111
22. Rodnina MV. Translation in Prokaryotes. Cold Spring Harb Perspect Biol. 2018;10(9):a032664. doi:10.1101/cshperspect.a032664
23. Ou M, Lin C, Wang Y, et al. Heterojunction engineered bioactive chlorella for cascade promoted cancer therapy. J Control Release. 2022;345:755–769. doi:10.1016/j.jconrel.2022.03.059
24. Qiao Y, Yang F, Xie T, et al. Engineered algae: a novel oxygen-generating system for effective treatment of hypoxic cancer. Sci Adv. 2020;6(21):eaba5996. doi:10.1126/sciadv.aba5996
25. Zhong D, Zhang D, Chen W, et al. Orally deliverable strategy based on microalgal biomass for intestinal disease treatment. Sci Adv. 2021;7(48):eabi9265. doi:10.1126/sciadv.abi9265
26. Zhang S, Xie F, Li K, et al. Gold nanoparticle-directed autophagy intervention for antitumor immunotherapy by inhibiting tumor-associated macrophage M2 polarization. Acta Pharm Sin B. 2022;12(7):3124–3138. doi:10.1016/j.apsb.2022.02.008
27. Zhong D, Li W, Qi Y, et al. Photosynthetic biohybrid nanoswimmers system to alleviate tumor hypoxia for FL/PA/MR imaging‐guided enhanced radio‐photodynamic synergetic therapy. Adv Funct Mater. 2020;30(17):1910395. doi:10.1002/adfm.201910395
28. Mertgen AS, Trossmann VT, Guex AG, et al. Multifunctional biomaterials: combining material modification strategies for engineering of cell-contacting surfaces. ACS Appl Mater Interfaces. 2020;12(19):21342–21367. doi:10.1021/acsami.0c01893
29. Zhuang D, He N, Khoo KS, et al. Application progress of bioactive compounds in microalgae on pharmaceutical and cosmetics. Chemosphere. 2022;291(Pt 2):132932. doi:10.1016/j.chemosphere.2021.132932
30. Chen Q-W, Qiao J-Y, Liu X-H, et al. Customized materials-assisted microorganisms in tumor therapeutics. Chem Soc Rev. 2021;50(22):12576–12615. doi:10.1039/d0cs01571g
31. Shannon E, Abu-Ghannam N. Antibacterial derivatives of marine algae: an overview of pharmacological mechanisms and applications. Mar Drugs. 2016;14(4):81. doi:10.3390/md14040081
32. El-Hack ME A, Abdelnour S, Alagawany M, et al. Microalgae in modern cancer therapy: current knowledge. Biomed Pharmacother. 2019;111:42–50. doi:10.1016/j.biopha.2018.12.069
33. ElFar OA, Billa N, Lim HR, et al. Advances in delivery methods of Arthrospira platensis (spirulina) for enhanced therapeutic outcomes. Bioengineered. 2022;13(6):14681–14718. doi:10.1080/21655979.2022.2100863
34. de la Asunción‐Nadal V, Franco C, Veciana A, et al. MoSBOTs: magnetically driven biotemplated MoS2‐based microrobots for biomedical applications. Small. 2022;18(33):2203821. doi:10.1002/smll.202203821
35. Wang H, Guo Y, Wang C, et al. Light-controlled oxygen production and collection for sustainable photodynamic therapy in tumor hypoxia. Biomaterials. 2021;269:120621. doi:10.1016/j.biomaterials.2020.120621
36. Zhong D, Li W, Hua S, et al. Calcium phosphate engineered photosynthetic microalgae to combat hypoxic-tumor by in situ modulating hypoxia and cascade radio-phototherapy. Theranostics. 2021;11(8):3580. doi:10.7150/thno.55441
37. Lee C, Lim K, Kim SS, et al. Chlorella-gold nanorods hydrogels generating photosynthesis-derived oxygen and mild heat for the treatment of hypoxic breast cancer. J Control Release. 2019;294:77–90. doi:10.1016/j.jconrel.2018.12.011
38. Zhou T-J, Xing L, Fan Y-T, et al. Light triggered oxygen-affording engines for repeated hypoxia-resistant photodynamic therapy. J Control Release. 2019;307:44–54. doi:10.1016/j.jconrel.2019.06.016
39. Dawiec-Liśniewska A, Podstawczyk D, Bastrzyk A, et al. aNew trends in biotechnological applications of photosynthetic microorganisms. Biotechnol Adv. 2022;59:107988. doi:10.1016/j.biotechadv.2022.107988
40. Delalat B, Sheppard VC, Rasi Ghaemi S, et al. Targeted drug delivery using genetically engineered diatom biosilica. Nat Commun. 2015;6(1):8791. doi:10.1038/ncomms9791
41. Todd T, Zhen Z, Tang W, et al. Iron oxide nanoparticle encapsulated diatoms for magnetic delivery of small molecules to tumors. Nanoscale. 2014;6(4):2073–2076. doi:10.1039/c3nr05623f
42. Zhang H, Shahbazi M-A, Mäkilä EM, et al. Diatom silica microparticles for sustained release and permeation enhancement following oral delivery of prednisone and mesalamine. Biomaterials. 2013;34(36):9210–9219. doi:10.1016/j.biomaterials.2013.08.035
43. Li M, Wu J, Lin D, et al. A diatom-based biohybrid microrobot with a high drug-loading capacity and pH-sensitive drug release for target therapy. Acta Biomater. 2022;154:443–453. doi:10.1016/j.actbio.2022.10.019
44. Maher S, Kumeria T, Wang Y, et al. From the mine to cancer therapy: natural and biodegradable theranostic silicon nanocarriers from diatoms for sustained delivery of chemotherapeutics. Adv Healthcare Mater. 2016;5(20):2667–2678. doi:10.1002/adhm.201600688
45. Simpi CC, Nagathan CV, Karajgi SR, et al. Evaluation of marine brown algae Sargassum ilicifolium extract for analgesic and anti-inflammatory activity. Pharmacognosy Res. 2013;5(3):146–149. doi:10.4103/0974-8490.112413
46. Bittkau KS, Dörschmann P, Blümel M, et al. Comparison of the effects of fucoidans on the cell viability of tumor and non-tumor cell lines. Mar Drugs. 2019;17(8):441. doi:10.3390/md17080441
47. Pang L, Jin H, Lu Z, et al. Treatment with mesenchymal stem cell‐derived nanovesicle‐containing gelatin methacryloyl hydrogels alleviates osteoarthritis by modulating chondrogenesis and macrophage polarization. Adv Healthcare Mater. 2023;12(17):2300315. doi:10.1002/adhm.202300315
48. Wang W, Zheng H, Jiang J, et al. Engineering micro oxygen factories to slow tumor progression via hyperoxic microenvironments. Nat Commun. 2022;13(1):4495. doi:10.1038/s41467-022-32066-w
49. Chen W, Ouyang J, Liu H, et al. Black phosphorus nanosheet-based drug delivery system for synergistic photodynamic/photothermal/chemotherapy of cancer. Adv Mater. 2017;29(5):1603864.
50. Qi F, Ji P, Chen Z, et al. Photosynthetic cyanobacteria‐hybridized black phosphorus nanosheets for enhanced tumor photodynamic therapy. Small. 2021;17(42):2102113. doi:10.1002/smll.202102113
51. Pereira AG, Otero P, Echave J, et al. Xanthophylls from the sea: algae as source of bioactive carotenoids. Mar Drugs. 2021;19(4):188. doi:10.3390/md19040188
52. Zaharescu T, Mateescu C. Investigation on some algal extracts as appropriate stabilizers for radiation-processed polymers. Polymers. 2022;14(22):4971. doi:10.3390/polym14224971
53. Vishchuk OS, Ermakova SP, Zvyagintseva TN. The fucoidans from brown algae of far-eastern seas: antitumor activity and structure‒function relationship. Food Chem. 2013;141(2):1211–1217. doi:10.1016/j.foodchem.2013.03.065
54. Kim EJ, Park SY, Lee J-Y, et al. Fucoidan present in brown algae induces apoptosis of human colon cancer cells. BMC Gastroenterol. 2010;10(1):1–11. doi:10.1186/1471-230X-10-96
55. Chen H, Cheng Y, Tian J, et al. Dissolved oxygen from microalgae-gel patch promotes chronic wound healing in diabetes. Sci Adv. 2020;6(20):eaba4311. doi:10.1126/sciadv.aba4311
56. Sun R, Chen H, Sutrisno L, et al. Nanomaterials and their composite scaffolds for photothermal therapy and tissue engineering applications. Sci Technol Adv Mater. 2021;22(1):404–428. doi:10.1080/14686996.2021.1924044
57. Martin LJ. Fucoxanthin and its metabolite fucoxanthinol in cancer prevention and treatment. Mar Drugs. 2015;13(8):4784–4798. doi:10.3390/md13084784
58. Silchenko AS, Rasin AB, Kusaykin MI, et al. Modification of native fucoidan from Fucus evanescens by recombinant fucoidanase from marine bacteria Formosa algae. Carbohydr Polym. 2018;193:189–195. doi:10.1016/j.carbpol.2018.03.094
59. Alekseyenko T, Zhanayeva SY, Venediktova A, et al. Antitumor and antimetastatic activity of fucoidan, a sulfated polysaccharide isolated from the Okhotsk Sea Fucus evanescens brown alga. Bull Exp Biol Med. 2007;143(6):730–732. doi:10.1007/s10517-007-0226-4
60. Huang T-H, Chiu Y-H, Chan Y-L, et al. Prophylactic administration of fucoidan represses cancer metastasis by inhibiting vascular endothelial growth factor (VEGF) and matrix metalloproteinases (MMPs) in Lewis tumor-bearing mice. Mar Drugs. 2015;13(4):1882–1900. doi:10.3390/md13041882
61. Koyanagi S, Tanigawa N, Nakagawa H, et al. Oversulfation of fucoidan enhances its anti-angiogenic and antitumor activities. Biochem Pharmacol. 2003;65(2):173–179. doi:10.1016/S0006-2952(02)01478-8
62. Yang L, Wang P, Wang H, et al. Fucoidan derived from Undaria pinnatifida induces apoptosis in human hepatocellular carcinoma SMMC-7721 cells via the ROS-mediated mitochondrial pathway. Mar Drugs. 2013;11(6):1961–1976. doi:10.3390/md11061961
63. Yan M-D, Yao C-J, Chow J-M, et al. Fucoidan elevates microRNA-29b to regulate DNMT3B-MTSS1 axis and inhibit EMT in human hepatocellular carcinoma cells. Mar Drugs. 2015;13(10):6099–6116. doi:10.3390/md13106099
64. Duan Y, Li J, Jing X, et al. Fucoidan induces apoptosis and inhibits proliferation of hepatocellular carcinoma via the p38 MAPK/ERK and PI3K/Akt signal pathways. Cancer Manag Res. 2020;12:1713. doi:10.2147/CMAR.S243495
65. Herschbein L, Liesveld JL. Dueling for dual inhibition: means to enhance effectiveness of PI3K/Akt/mTOR inhibitors in AML. Blood Rev. 2018;32(3):235–248. doi:10.1016/j.blre.2017.11.006
66. Cikutović-Molina R, Herrada AA, González W, et al. TASK-3 gene knockdown dampens invasion and migration and promotes apoptosis in KATO III and MKN-45 human gastric adenocarcinoma cell lines. Int J Mol Sci. 2019;20(23):6077. doi:10.3390/ijms20236077
67. Carmichael W. Cyanobacteria secondary metabolites—the cyanotoxins. J App Bacteriol. 1992;72(6):445–459. doi:10.1111/j.1365-2672.1992.tb01858.x
68. Stevenson CS, Capper EA, Roshak AK, et al. The identification and characterization of the marine natural product scytonemin as a novel antiproliferative pharmacophore. J Pharmacol Exp Ther. 2002;303(2):858–866. doi:10.1124/jpet.102.036350
69. Kerbrat P, Dieras V, Pavlidis N, et al. Phase II study of LU 103793 (dolastatin analog) in patients with metastatic breast cancer. Eur J Cancer. 2003;39(3):317–320. doi:10.1016/S0959-8049(02)00531-2
70. Hong J, Luesch H. Largazole: from discovery to broad-spectrum therapy. Nat Prod Rep. 2012;29(4):449–456. doi:10.1039/c2np00066k
71. Wang Z, Gong X, Li J, et al. Oxygen-delivering polyfluorocarbon nanovehicles improve tumor oxygenation and potentiate photodynamic-mediated antitumor immunity. ACS nano. 2021;15(3):5405–5419. doi:10.1021/acsnano.1c00033
72. Kessel D. Photodynamic therapy: critical PDT Theory. Photochem Photobiol. 2023;99(2):199–203.
73. Matsumoto Y, Cao E, Ueoka R. Growth inhibition by novel liposomes including trehalose surfactant against hepatocarcinoma cells along with apoptosis. Anticancer Res. 2013;33(11):4727–4740.
74. Shchelik IS, Sieber S, Gademann K. Green algae as a drug delivery system for the controlled release of antibiotics. Chem–A Euro J. 2020;26(70):16644–16648. doi:10.1002/chem.202003821
75. Weibel DB, Garstecki P, Ryan D, et al. Microoxen: microorganisms to move microscale loads. Proc Natl Acad Sci. 2005;102(34):11963–11967. doi:10.1073/pnas.0505481102
76. Witman GB. Chlamydomonas phototaxis. Trends Cell Biol. 1993;3(11):403–408. doi:10.1016/0962-8924(93)90091-E
77. Losic D, Yu Y, Aw MS, et al. Surface functionalisation of diatoms with dopamine modified iron-oxide nanoparticles: toward magnetically guided drug microcarriers with biologically derived morphologies. Chem Comm. 2010;46(34):6323–6325. doi:10.1039/c0cc01305f
78. Zhang D, Zhong D, Ouyang J, et al. Microalgae-based oral microcarriers for gut microbiota homeostasis and intestinal protection in cancer radiotherapy. Nat Commun. 2022;13(1):1413. doi:10.1038/s41467-022-28744-4
79. Li W, Zhong D, Hua S, et al. Biomineralized biohybrid algae for tumor hypoxia modulation and cascade radio-photodynamic therapy. ACS Appl Mater Interfaces. 2020;12(40):44541–44553. doi:10.1021/acsami.0c14400
80. Liu Z, Fu X, Huang W, et al. Photodynamic effect and mechanism study of selenium-enriched phycocyanin from Spirulina platensis against liver tumors. J Photochem Photobiol B. 2018;180:89–97. doi:10.1016/j.jphotobiol.2017.12.020
81. Watanabe A, Minagawa J. Structural characterization of the photosystems in the green alga Chlorella sorokiniana. Planta. 2020;252(5):1–6. doi:10.1007/s00425-020-03487-y
82. Ghosh P, Vaidya A, Sahoo R, et al. Glycation of the complement regulatory protein CD59 is a novel biomarker for glucose handling in humans. J Clin Endocrinol Metab. 2014;99(6):E999–e1006. doi:10.1210/jc.2013-4232
83. Yasa O, Erkoc P, Alapan Y, et al. Microalga-powered microswimmers toward active cargo delivery. Adv Mater. 2018;30(45):e1804130. doi:10.1002/adma.201804130
84. Wang H, Liu H, Guo Y, et al. Photosynthetic microorganisms coupled photodynamic therapy for enhanced antitumor immune effect. Bioact Mater. 2022;12:97–106. doi:10.1016/j.bioactmat.2021.10.028
85. Chiaravalloti A, Rubello D, Chondrogiannis S, et al. Low-dose CT and contrast-medium CT in hybrid PET/CT systems for oncologic patients. Nucl Med Commun. 2015;36(9):867–870. doi:10.1097/MNM.0000000000000314
86. Sotoudeh H, Sharma A, Fowler KJ, et al. Clinical application of PET/MRI in oncology. J Magn Reson Imaging. 2016;44(2):265–276. doi:10.1002/jmri.25161
87. Gargiulo S, Albanese S, Mancini M. State-of-the-art preclinical photoacoustic imaging in oncology: recent advances in cancer theranostics. Contrast Media Mol Imaging. 2019;2019:1–24. doi:10.1155/2019/5080267
88. Silva A, Silva SA, Carpena M, et al. Macroalgae as a source of valuable antimicrobial compounds: extraction and applications. Antibiotics. 2020;9(10):642.
89. Zou CY, Lei XX, Hu JJ, et al. Multicrosslinking hydrogels with robust bioadhesion and pro-coagulant activity for first-aid hemostasis and infected wound healing. Bioact Mater. 2022;16:388–402. doi:10.1016/j.bioactmat.2022.02.034