Infection
Superbugs a catastrophe in the making for Canadian hospitals, doctor says
This story is part of CBC Health’s Second Opinion, a weekly analysis of health and medical science news emailed to subscribers on Saturday mornings. If you haven’t subscribed yet, you can do that by clicking here.
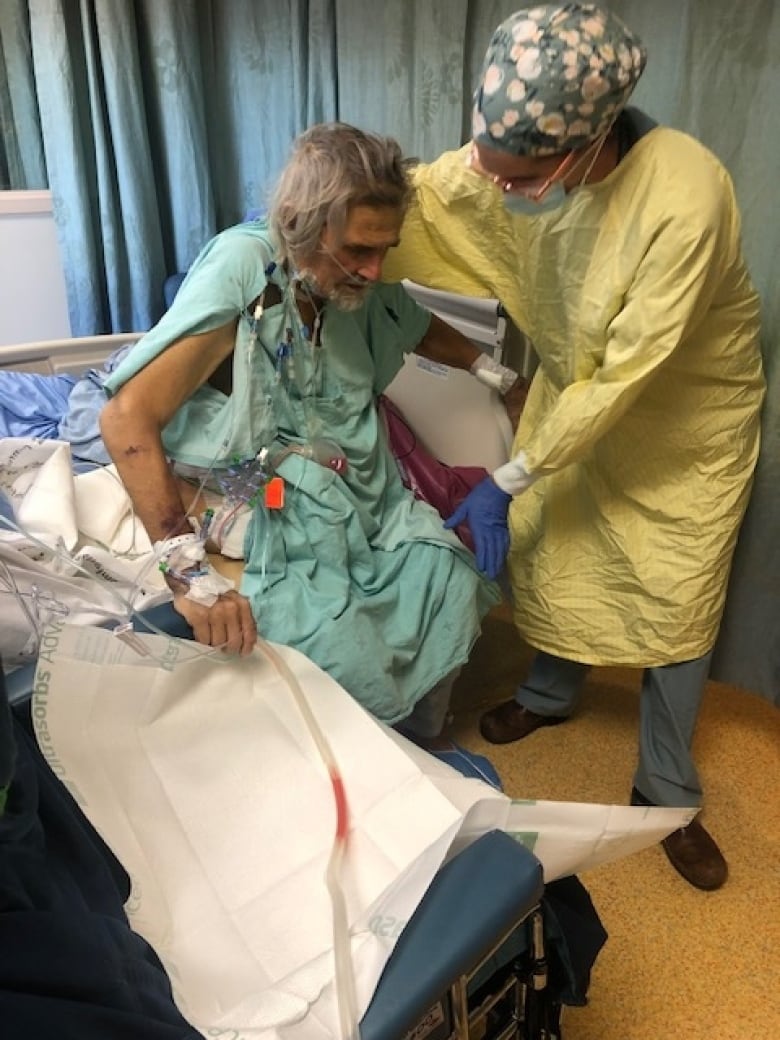
Glenn Barr was returning from work one long weekend a few years ago, when he suddenly felt terrible.
The Ottawa resident was soon vomiting blood onto his driveway. A trip to his nurse practitioner and then the emergency department eventually led to a diagnosis of end-stage cirrhosis of the liver, landing him on the transplant list.
After waiting four years to qualify and find a match, this Labour Day marked the second anniversary of his liver transplant. His medical teams were never able to determine what caused his liver damage.
But another part of the transplantation experience that caught him off guard were the half-dozen, hard-to-treat infections he endured. Barr faced fever, aches and diarrhea from the infections, both before and after the surgery.
“I was incredibly sick,” Barr recalled. “The doctors would open up my charts, and if it was a new doctor, you’d hear, ‘Oh my. Oh Glenn.'”
Barr, a 67-year-old electrical contractor, needed many blood transfusions for internal bleeding and a series of procedures to get through the transplant and its complications, including an incision infection that couldn’t be seen on the skin.
Doctors had to cut out the infected tissue and give antibiotics through an IV.
“They wouldn’t let me out of the hospital for five weeks, until they were happy that the blood work that they saw was good,” he said.
Increasingly, physicians worry that infections that typically kill people with weakened immune systems will expand to hit Canadians going in for routine surgery, especially as cases of drug-resistant bacterial and fungal pathogens become more common.
Drug or antimicrobial resistance occurs when bacteria, viruses or fungi evolve over time and ultimately stop responding to the treatments that once killed them, making infections harder to treat. Also called superbugs, the World Health Organization has declared these pathogens an urgent global public health threat.
According to a study published in The Lancet medical journal, they killed at least an estimated 1.27 million people worldwide in 2019 alone. And in the U.S., the Centers for Disease Control and Prevention (CDC) reports� more than 2.8 million antimicrobial-resistant infections each year.
Deaths from impossible to treat infections
For infectious disease physicians like Dr. Ilan Schwartz, the concern is that people coming to hospital for scheduled surgeries will also acquire infections that are untreatable or extremely difficult to control.
Superbugs threaten much of our modern medicine because they’re resistant to the antibiotics used during routine surgeries or treatments, like C-sections, cancer care and joint replacements. Hospitals are rife with opportunities for potential exposure, including surgical cuts or the use of IVs, ventilators or catheters.
The infections can prolong a patient’s hospital stay for weeks or months, adding to backlogs in already-clogged systems.

“We already do have patients in Canada that are dying of untreatable infection,” said Schwartz, who previously worked in Edmonton but moved to Duke University, in Durham, N.C., last year.
“In this arm’s race against the bacteria, we inevitably lose.”
Gerry Wright, a professor of biochemistry and biomedical studies, works to develop new antibiotics at his lab at McMaster University. For Wright, the trillions of bacteria have the upper hand, thanks to how quickly they reproduce to swap DNA and evolve to gain resistance.
Wright said antibiotics saved his life when a “rumbly tummy” from a foodborne illness years ago in Europe got into his bloodstream and wreaked havoc. When Wright returned to Canada, the bacteria were resistant to oral antibiotics. Like Barr, he needed an IV to treat the infection.
Bacteria with a ‘harder’ M&M shell
One class of bacteria known as Gram negatives pose a particular threat, physicians and microbiologists say.
Wright compared Gram positive bacteria like Staphylococcus aureus, to a plain M&M candy, with a thin coating for antibiotics to penetrate. On the other hand, Gram negative bacteria are like peanut M&Ms, he said, with a much harder shell.
Schwartz agrees. “I think the Gram negatives are what is going to ultimately lead to a catastrophe in health care.”
Wright also suggests the rapid spread of COVID-19 should serve as a wake-up call, showing how quickly pathogens without treatment options can spread.
“We had an enormous lesson given to us — a spanking by nature from COVID,” Wright said. “And instead of learning our lesson, I’m very concerned that what we want to do is completely forget the embarrassment that we got caught unprepared.”
Fungal slime poses particular threat
Globally, experts are also particularly worried about one drug-resistant fungal species, Candida auris.
First identified in Japan in 2009, C. auris are widespread overseas and quickly becoming entrenched in some U.S. states. The CDC estimates that cases of infection (and simply carrying the fungi without symptoms) has tripled in recent years, rising from 476 in 2019, to 1,471 in 2021.
Part of the fungus’s hardiness is thanks to the fact it is encased in slime that protects it from disinfectants. The slime, called a biofilm, makes it tough to stamp out in hospitals and long-term care homes. Plus, fungi also pass easily from one patient to another. When it invades the body, it can be hard to treat.

To take Wright’s M&M coating metaphor a step further, the shells on fungi are even thicker than what’s found on bacteria — more like a watermelon rind.
Schwartz helped document when C. auris first landed in Canada in 2012 — though it is currently less prevalent here than in the U.S. It was introduced when a man from India was transferred to hospital in Manitoba and fluid from his ear infection was found to contain the fungi.
As of Sept. 6, the Public Health Agency of Canada (PHAC) said 48 cases of C. auris have been reported across this country since the first case was identified. In a sign of its rise, though, 31 of the 48 cases have been found since 2019.
Spread the word, not the germ
Schwartz said Canada has dodged a bullet by not having more resistant cases of the fungus. He attributes it to:
- Having fewer highly sick patients at specialized nursing homes than in the U.S.
- Careful screening of patients hospitalized from countries known to be at high risk for drug-resistant bacteria and fungi.
- Plain good luck.
Scientists have several lines of thinking on why Candida auris arose in several places in the world all at once, including global warming, overuse of drugs like antibiotics and environmental changes.

Fungal expert Shawn Lockhart favours changes in the microbiome — the sum of all the bacteria, viruses and fungi in and on us — as the reason behind the rise.
He points to a study by scientists at the U.S. National Institutes of Health who compared patients with similar conditions who had C. auris to those who didn’t. People with the fungus showed complete changes in the microbiome of their skin, such as switching from Gram positive to Gram negative.
“That’s a clue that these changes in the microbiome allow it to emerge,” said Lockhart, who is a senior clinical laboratory advisor at the CDC’s mycotic diseases branch in Atlanta.
Drug-resistant microbes, like bacteria and fungi, tend to travel in the same circles, Schwartz said, including in health-care settings around the world, where misuse and overuse of antibiotics and antifungals is rampant.
He’s also concerned the increased use of the drugs outside of medicine, such as in livestock agriculture, can also foster microbial resistance to human medications.

Schwartz compared antibiotics to fire extinguishers, in that both should be held in reserve. “We don’t want to reach for them until we absolutely have to.”
Pharmaceutical companies haven’t created many new antibiotics in years because there’s little incentive for them, Schwartz said.
What’s more, getting new antibiotics to market anywhere in the world is a challenge because they need to work really well, all at once, without harming us.
Infectious disease doctors in Canada in particular have their hands tied when reaching for the newest antibiotics compared with their U.S. counterparts, noted Schwartz. Last week, the Council of Canadian Academies released a report, Overcoming Resistance, on encouraging pharmaceutical companies to make high-value drugs available in this country.
Until then, the Canadian focus is on keeping superbugs out of vulnerable settings, like hospitals and long-term care, wherever possible.