Cardiovascular
Cardiologists in China are world’s first to use new annuloplasty system for tricuspid regurgitation
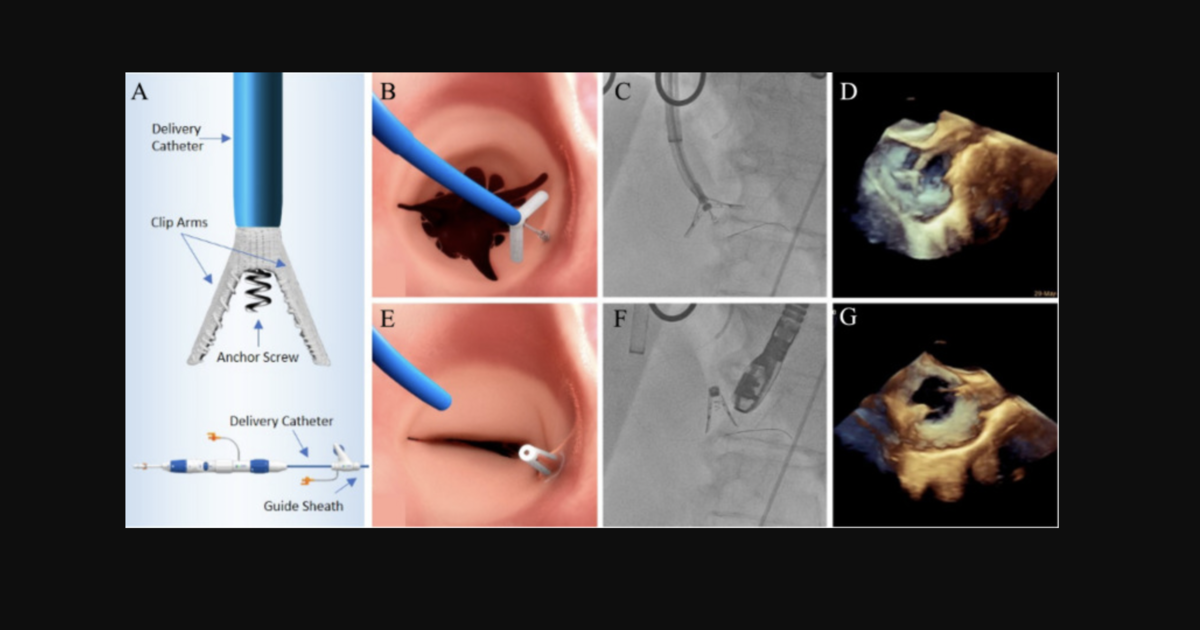
A brand new device: Meet the K-Clip annuloplasty system for tricuspid regurgitation
Zhang et al. focused on the first 15 patients treated with the K-Clip annuloplasty system, designed and developed by Shanghai Huihe Medical Technology Co. The researchers described the device as a “transcatheter tricuspid valve repair device via transjugular venous access for direct annulus reduction.” Its design includes two clamp arms and one anchor screw, which combine to form what they called a “clamp-annular-screw sandwich structure.” The K-Clip system was designed with four arm lengths to choose from—12 mm, 14 mm, 16 mm and 18 mm—and is implanted with help from a 15F delivery catheter.
All 15 patients presented with severe TR and received treatment at Zhongshan Hospital between March 2021 and February 2022. The mean patient age was just shy of 73 years old, and 60% of patients were male. The most common comorbid conditions were atrial fibrillation (93%) and hypertension (73%). Procedures were performed under general anesthesia using transesophageal echocardiography and fluoroscopy. A majority of patient were prescribed dual-antiplatelet therapy for a minimum of one month following hospital discharge.
Overall, the procedural success rate was 100%. No major adverse cardiovascular events were reported after 30-day follow-up. One patient did experience mild bleeding, but no invasive interventions were required to resolve the issue.
In addition, the authors noted, 30-day echocardiography revealed “remarkable” reductions in tricuspid annular circumference and area. One in three patients still presented with TR that was greater than moderate after 30 days, but the mean Kansas City Cardiomyopathy Questionnaire score improved from 62.28 to 77.90.
Overall, the research team highlighted the K-Clip’s “high technical success,” “acceptable safety” and “significant clinical improvements.” However, they wrote, large studies with long-term follow-up are still necessary to learn more about the device’s impact on patients presenting with severe TR.
Read the full study here.