Cancer and neoplasms
Comparison of diagnostic values of ACR TI-RADS v/s C-TIRADS
Thyroid cancer is the most common endocrine tumor and the 11th most common malignant tumor worldwide.1 Moreover, thyroid cancer is also one of the malignancies with the highest increasing rate, ie, a 3-fold increase in incidence has been observed during the past 3 decades, with an increasing global annual rate of 3.6%.2 Thyroid cancer has good overall prognosis and sees a > 90% 5-year survival rate.3 However, elderly patients who are elderly have the greatest risk of mortality and morbidity in this age group. It has been shown that this age group costs the health service a significant amount of revenue per annum for what should be considered a relatively rare malignancy.4,5
Thyroid Imaging Reporting and Data System (TI-RADS) has been used for the malignancy stratification of thyroid nodules, thereby providing imaging evidence for the clinical diagnosis and treatment.6–9 The American College of Radiology (ACR) Thyroid Imaging Reporting and Data System (TI-RADS) and Chinese Thyroid Imaging Reporting and Data System (C-TIRADS) are the two most authoritative ultrasound thyroid nodule classification systems, both of which have been widely used in China. However, which TI-RADS system is more suitable for elderly patients with thyroid nodules has not yet been reported.
In this study, the ultrasound features of 512 elderly thyroid nodules were analyzed to explore the diagnostic value of the C-TIRADS classification system for elderly thyroid cancer.
Materials and Methods
General Information
All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of the Affiliated Hospital of Jiangnan University. Individual consent for this retrospective analysis was waived. A total of 465 elderly patients with surgical pathology-proven thyroid nodules were included in the Affiliated Hospital of Jiangnan University, General Hospital of Eastern Theater Command (the former Bayi Hospital), and Southeast University Affiliated Zhongda Hospital between January 2021 and December 2022. The major indications for surgery included the following: 1) confirmed or suspected with malignant thyroid tumor according to the fine needle aspiration (FNA) findings; 2) with no clear pathological result, but the nodule was highly suspicious based on ultrasound findings and was >1 cm; 3) the nodule was ≤1 mm and the FNA result was unknown, but the ACR TI-RADS or C-TIRADS classification of the nodule was ≥4; 4) patient was extremely anxious and required surgical treatment; 5) the nodule was very large and caused compression symptoms of surrounding tissues.
Apparatus and Methods
The S60 color Doppler ultrasonic imaging machine and linear array probe with a 7.8–15 MHz frequency were used. The patient was placed in the supine position, the bilateral sides of the neck were fully exposed, and the combination of cross-sectional and longitudinal scanning was used for the thyroid gland scanning, and the thyroid nodules within the range were observed from multiple sections and angles. To evaluate the nodules, the ACR TI-RADS and C-TIRADS scoring systems were used simultaneously by two attending or higher sonographers (with more than 10 years’ working experience) independently. In case of disagreements, a chief physician was invited.
ACR TI-RADS Score and Classification
According to the ACR TI-RADS system,8 the criteria of ultrasound evaluation and ACR TI-RADS scoring and classification are showed in Table 1 and Table 2.
 |
Table 1 Five Ultrasonic Features and Corresponding Scores in ACR TI-RADS Classification |
 |
Table 2 The ACR TI-RADS Based on the Counting Method |
C-TIRADS Score and Classification
According to the C-TIRADS system developed by the counting method,9 the criteria of ultrasound evaluation and C-TIRADS scoring and classification are showed in Table 3 and Table 4.
 |
Table 3 Ultrasonic Positive and Negative Features and Corresponding Scores |
 |
Table 4 The C-TIRADS Based on the Counting Method |
Statistical Analysis
SPSS 27.0 and MedCalc19.3.1 software were used to calculate the study size and the statistical analysis. Quantitative data with normal distribution were described by mean ± standard deviation and compared by independent t-test. Quantitative data with non-normal distribution were described by M (P25, P75) and compared by the Mann–Whitney U-test. Qualitative data were described by frequency and percentage and compared by χ2 or Fisher Exact tests. Surgical pathological findings were used as the gold standard; the receiver operating characteristic curve (ROC) was plotted, and the area under the curve (AUC) and Youden index were calculated. The AUC of 0.85–0.95 indicated high diagnostic efficacy, the AUC of 0.70–0.85 indicated fair diagnostic efficacy, and the AUC of 0.50–0.70 indicated poor diagnostic efficacy. The scores of the groups at the highest Youden index were considered the best cut-off value of the scores. Z test was used for the comparison of AUC. P<0.05 was considered statistically significant.
Results
Pathological Results
A total of 512 nodules from 465 elderly patients with surgical pathology-proven thyroid nodules were included. According to the 5th edition of the WHO classification of tumors of endocrine organs (Thyroid),10,11 512 elderly thyroid nodules included 254 malignant nodules with an incidence rate of 49.61%, 254 benign nodules with an incidence rate of 49.61%, and 4 low-risk nodules with an incidence rate of 0.78%. The pathological results of the thyroid nodules in different groups are distributed as shown in Table 5. Among these nodules, nodular goiter is the main type of benign thyroid nodules, and papillary thyroid carcinoma is the main type of malignant thyroid nodules.
 |
Table 5 Pathological results of the elderly thyroid nodules |
Ultrasound Features and C-TIRADS Positive Indicators of Benign and Malignant Thyroid Nodules
In order to facilitate the analysis, the low-risk thyroid nodules were classified as malignant nodules in this study. The ultrasound features of benign and malignant thyroid nodules in elderly patients are shown in Table 6, and the composition, echogenicity, shape, margin, and echogenic foci of benign and malignant thyroid nodules were all statistically significantly different (all P<0.05). The positive indicator classifications of C-TIRADS are shown in Table 7, and the classifications of malignant nodules were significantly higher than benign nodules (all P<0.05). The typical benign and malignant thyroid nodules in the elderly are shown in Figures 1 and 2.
 |
Table 6 Ultrasonic Features of Benign and Malignant Thyroid Nodules (n) |
 |
Table 7 C-TIRADS Positive Indicators of Benign and Malignant Thyroid Nodules (n) |
|
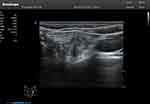
Figure 1 72-year-old man with thyroid papillary carcinoma. |
|
Figure 2 63-year-old woman with nodular goiter. |
Comparison of ACR TI-RADS versus C-TIRADS Scoring and Classification Systems for Thyroid Nodules
In this study, the mean ACR TI-RADS score of the 1599 thyroid nodules was 6.53 ± 3.45 (0–14), and the mean C-TIRADS score was 2.19 ± 1.61 (0–5). The ACR TI-RADS and C-TIRADS scores for benign nodules were both significantly lower than malignant nodules (all P < 0.05) (Table 8). The nodules were classified according to the ACR TI-RADS and C-TIRADS scores and then compared with the pathological results. The number of benign and malignant thyroid nodules of different types of nodules is shown in Table 9. The ACR TI-RADS and C-TIRADS classifications of malignant nodules were significantly higher than benign ones (P<0.05). The typical ultrasound images of benign and malignant thyroid nodules are shown in Figures 1 and 2.
 |
Table 8 Comparison of ACR TI-RADS versus C-TIRADS Scores of Elderly Thyroid Nodules |
 |
Table 9 ACR TI-RADS and C-TIRADS Classifications of Elderly Thyroid Nodules |
ROC Curves and Diagnostic Efficacies of ACR TI-RADS and C-TIRADS Scoring and Classification Systems
The ROC curve was plotted based on the scoring and classification results of benign and malignant thyroid nodules (Figure 3). The respective AUC of ACR TI-RADS scoring and classification system was 0.861 (95% CI: 0.828 to 0.890) and 0.897 (95% CI: 0.867 to 0.922) (Z = 4.403, P 6 and >TR 4. The respective AUC of the C-TIRADS scoring and classification system was 0.879 (95% CI: 0.848 to 0.906) and 0.900 (95% CI: 0.870 to 0.924) (Z = 3.653, 0.0003). When the Youden index was the highest, the respective best cut-off value of the C-TIRADS scoring and classification system was >2 and >C-TR 4B. The AUC of ACR TI-RADS scores and C-TIRADS scores were not significantly different (Z = 0.350, P = 0.7263 > 0.05), while the AUC of ACR TI-RADS classifications was significantly lower than C-TIRADS classifications (Z = 1.944, P = 0.0519 > 0.05).
 |
Figure 3 ROC curves of ACR TI-RADS and C-TIRADS score and classification. (A) shows diagnostic efficiency of ACR TI-RADS score and classification system for the elderly thyroid cancers; (B) shows diagnostic efficiency of C-TIRADS score and classification system for the elderly thyroid cancers. |
The indicators reflecting the diagnostic efficacies of ACR TI-RADS and C-TIRADS scoring and classification systems for elderly thyroid nodules are shown in Table 10. The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of the ACR TI-RADS scoring and classification systems were 89.53%, 78.35%, 80.77%, and 88.05%, respectively. The sensitivity, specificity, PPV, and NPV of the C-TIRADS scoring and classification systems were 86.05%, 81.10%, 82.22%, and 85.12%, respectively. The diagnostic efficacies of ACR TI-RADS and C-TIRADS scoring and classification systems were not significantly different (all P values >0.05).
 |
Table 10 Diagnostic Efficacy of the ACR TI-RADS and C-TIRADS Scoring and Classification Systems |
Discussion
The results of an epidemiological survey of 78,470 subjects from 31 provinces and cities in China showed that the overall prevalence of thyroid disease was 50.96%, which was higher in the elderly than in the general population.12 Previous studies have also shown that age is an independent risk factor for the prognosis of thyroid cancer.13 Most thyroid cancers have good prognosis; however, due to the distinctively different two gene mutations in elderly and young patients,14 the risk of recurrence and metastasis of thyroid cancer is linearly related to age; also, the survival rate decreases with age and vascular invasion and early metastasis are more common in elderly patients.15,16 Therefore, it is important to assess the diagnostic value of current classification systems for elderly thyroid cancer. The findings of this study showed that both ACR-TIRADS and C-TIRADS have high diagnostic efficacies for elderly thyroid cancer. The results of this study showed that both ACR-TIRADS and C-TIRADS had good diagnostic efficacy for thyroid cancer in the elderly, and the sensitivity, specificity, positive predictive value, and negative predictive value are high as well. However, the ACR TI-RADS score and classification system correspond to the optimal thresholds of >6 points and >4 categories, and the C-TIRADS score and classification system correspond to the optimal thresholds of >2 points and >4B categories, which may increase the underdiagnosis of elderly thyroid cancer to some extent.
A total of 512 elderly thyroid nodules were analyzed in this study. The classification analysis showed that although the classification of malignant nodules was higher than benign nodules, there were still overlaps between the ACR TI-RADS and C-TIRADS classifications of benign and malignant nodules. The benign nodules included nodular goiter, follicular adenoma, and lymphocytic thyroiditis. Previous studies found evident overlaps between the ultrasound features of lymphocytic thyroiditis and PTMC,17,18 which could be easily misdiagnosed to a certain extent. However, for most nodular goiters, the typical ultrasound features included solid-cystic, clear margin, and wider-than-tall.16 However, the ultrasound features of some nodular goiters in this study were similar to PTMC, which could easily result in misdiagnosis. Previous studies have shown that such nodules mostly degenerate benign thyroid nodules caused by bleeding, degeneration, infarction, or fibrosis. During the process of hematoma absorption, the degenerated thyroid nodules gradually became irregular with the shrinking of the nodules, which also showed malignant ultrasound features.19,20 Furthermore, when the nodular goiters are accompanied by interstitial collagenization, calcification, and crystal formation, some nodular goiters could also show the accompaniment of punctate echogenic foci with the elapse of time. However, in solid thyroid micronodules, it is difficult to accurately distinguish such nodules from microcalcifications.21 In addition, due to the small size of the nodules, the probability of cystic change is very low, which increases the difficulty of distinguishing them from thyroid cancer to a certain extent.
The analysis of ROC curves showed that the AUC of ACR TI-RADS and C-TIRADS scoring and classification systems was 0.861, 0.897, 0.879, and 0.900, respectively, which was between 0.85 and 0.95, indicating that both the ACR TI-RADS and C-TIRADS scoring systems had high diagnostic efficacies for elderly thyroid nodules, which is consistent with the results reported by Jianqiao et al, who reported an AUC of 0.890.8 In the study by Qi et al,22 who did not stratify groups by age, the AUC of ACR TI-RADS and C-TIRADS classification systems for diagnosing malignant thyroid nodules were 0.937 and 0.854, respectively, which is in agreement with the AUC of this study, indicating that the diagnostic efficacies of the ACR TI-RADS and C-TIRADS classification systems for elderly thyroid cancer might not be affected by age. The best cut-off value of the ACR TI-RADS and C-TIRADS score were >6 and >4 points, and the best cut-off value of the classification was > TR 4 and C-TR 4B, respectively, which was in agreement with the findings reported by Kang et al and Hu et al.23,24 However, in the course of clinical practice, taking ACR TI-RADS >4 and C-TIRADS>4B as the optimal threshold will lead to the missed diagnosis of some cases. Especially for patients who require organ transplantation, thyroid nodules in potential organ donors need to be investigated to rule out cancer and avert any subsequent risk of transmission. If the donor is an elderly population with thyroid nodules, the misdiagnosis of thyroid cancer may have certain risks to organ transplant recipients.25 Meanwhile, the sensitivity is 86.05% at this time, which is significantly lower than that corresponding to taking ACR TI-RADS >3 and C-TIRADS>3 as the optimal threshold, although the specificity and accuracy are both higher. In view of the higher aggressiveness, as well as metastasis and recurrence rates of thyroid cancer in the elderly, the clinical diagnosis and treatment strategy of thyroid nodules with ACR TI-RADS >3 category and C-TIRADS>3 category can be appropriately adjusted for elderly patients, so as to avoid the misdiagnosis of thyroid cancer. Therefore, the authors believe that there is room for further optimization of both ACR TI-RADS and C-TIRADS for elderly thyroid cancer. Especially with the development of artificial intelligence technology in recent years, artificial intelligence can assist physicians in diagnosis thyroid nodules, improve diagnostic accuracy, reduce diagnostic bias caused by human factors and deserves a comment as maybe future direction for elderly thyroid cancer as well.26,27
Limitations
The present study assessed the diagnostic efficacies of ACR TI-RADS and C-TIRADS scoring and classification systems for elderly thyroid cancer, using the surgical pathological findings as the reference. However, due to the strict indications for surgery, only 512 elderly thyroid nodules were included in this study. Also, under the background of over-diagnosis and –treatment of thyroid microcarcinoma, the 512 surgical pathology-proven thyroid nodules still contained a substantial proportion of thyroid micronodules, which could bias the results of this study to a certain extent.
Conclusions
ACR TI-RADS and C-TIRADS scoring and classification systems have high diagnostic efficacy for elderly thyroid cancer, which has high clinical practical values, but there is still a significant risk of missed diagnosis when taking ACR TI-RADS >4 and C-TIRADS>4B as the optimal threshold.
Ethical Statement
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Affiliated Hospital of Jiangnan University. Individual consent for this retrospective analysis was waived. For the patient’s information provided by the Affiliated Hospital of Jiangnan University during the treatment period due to illness, such as name, age, gender, occupation, address, ID card, related diseases and treatment plan. Due to the privacy of patients, the Affiliated Hospital of Jiangnan University keeps the above information confidential.
Funding
This study was supported by the Wuxi Maternal and Child Health Promotion Project (FYTG202103).
Disclosure
The authors declare that they have no conflicts of interest for this work.
References
1. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi:10.3322/caac.21492
2. Lim H, Devesa SS, Sosa JA, et al. Trends in thyroid cancer incidence and mortality in the United States, 1974–2013. JAMA. 2017;317:1338–1348. doi:10.1001/jama.2017.2719
3. Seib CD, Sosa JA. Evolving understanding of the epidemiology of thyroid cancer. Endocrinol Metab Clin North Am. 2019;48(1):23–35. doi:10.1016/j.ecl.2018.10.002
4. Gosain R, Alexander JS, Gill A, et al. Radioactive iodine-refractory differentiated thyroid cancer in the elderly. Curr Oncol Rep. 2018;20:82. doi:10.1007/s11912-018-0736-4
5. Wang J, Zhanghuang C, Jin L, et al. Development and validation of a nomogram to predict cancer-specific survival in elderly patients with papillary thyroid carcinoma: a population-based study. BMC Geriatr. 2022;22(1):736. doi:10.1186/s12877-022-03430-8
6. Ha EJ, Chung SR, Na DG, et al. Korean thyroid imaging reporting and data system and imaging-based management of thyroid nodules: Korean Society of Thyroid Radiology consensus statement and recommendations. Korean J Radiol. 2021;22:2094–2123. doi:10.3348/kjr.2021.0713
7. Russ G, Bonnema SJ, Erdogan MF, et al. European Thyroid Association Guidelines for ultrasound malignancy risk stratification of thyroid nodules in adults: the EU-TIRADS. Eur Thyroid J. 2017;6:225–237. doi:10.1159/000478927
8. Tessler FN, Middleton WD, Grant EG, et al. ACR Thyroid Imaging, Reporting and Data System (TI-RADS): white paper of the ACR TI-RADS Committee. J Am Coll Radiol. 2017;14:587–595. doi:10.1016/j.jacr.2017.01.046
9. Zhou J, Yin L, Wei X, et al. Chinese guidelines for ultrasound malignancy risk stratification of thyroid nodules: the C-TIRADS. Endocrine. 2020;70:256–279. doi:10.1007/s12020-020-02441-y
10. Mete O, Wenig BM. Update from the 5th Edition of the World Health Organization Classification of head and neck tumors: overview of the 2022 WHO classification of head and neck neuroendocrine neoplasms. Head Neck Pathol. 2022;16(1):123–142. doi:10.1007/s12105-022-01435-8
11. Baloch ZW, Asa SL, Barletta JA, et al. Overview of the 2022 WHO classification of thyroid neoplasms. Endocr Pathol. 2022;33:27–63. doi:10.1007/s12022-022-09707-3
12. Li Y, Teng D, Ba J, et al. Efficacy and safety of long-term universal salt iodization on thyroid disorders: epidemiological evidence from 31 Provinces of Mainland China. Thyroid. 2020;30(4):568–579. doi:10.1089/thy.2019.0067
13. Adam MA, Thomas S, Hyslop T, et al. Exploring the relationship between patient age and cancer-specific survival in papillary thyroid cancer: rethinking current staging systems. J Clin Oncol. 2016;34:4415–4420. doi:10.1200/JCO.2016.68.9372
14. Prete A, Borges de Souza P, Censi S, et al. Update on fundamental mechanisms of thyroid cancer. Front Endocrinol. 2020;13:102. doi:10.3389/fendo.2020.00102
15. Samra B, Jabbour E, Ravandi F, et al. Evolving therapy of adult acute lymphoblastic leukemia: state-of-the-art treatment and future directions. J Hematol Oncol. 2020;13(1):70. doi:10.1186/s13045-020-00905-2
16. Vini L, Hyer SL, Marshall J, et al. Long-term results in elderly patients with differentiated thyroid carcinoma. Cancer. 2003;97:2736–2742. doi:10.1002/cncr.11410
17. Yang L, Zhao H, He Y, et al. Contrast-enhanced ultrasound in the differential diagnosis of primary thyroid lymphoma and nodular hashimoto’s thyroiditis in a background of heterogeneous parenchyma. Front Oncol. 2021;7:597–603. doi:10.3389/fonc.2020.597975
18. Zhao W, Kang Q, Qian F, et al. Convolutional neural network-based computer-assisted diagnosis of hashimoto’s thyroiditis on ultrasound. J Clin Endocrinol Metab. 2022;107(4):953–963. doi:10.1210/clinem/dgab870
19. Yan Y, Zhang F, Ge H, et al. Effect of the size of benign thyroid degenerative nodules on ACR TI-RADS categories. J Med Ultrason. 2001;2022(9):71–76. doi:10.1007/s10396-021-01163-6
20. Huang QX, Huang XW. Comments on “Effect of the size of benign thyroid degenerative nodules on ACR TI-RADS categories”. J Med Ultrason. 2001;2022(49):503. doi:10.1007/s10396-022-01214-6
21. Choi WJ, Park JS, Kim KG, et al. Computerized analysis of calcification of thyroid nodules as visualized by ultrasonography. Eur J Radiol. 2015;84(10):1949–1953. doi:10.1016/j.ejrad.2015.06.021
22. Qi Q, Zhou A, Guo S, et al. Explore the diagnostic efficiency of Chinese Thyroid Imaging Reporting and Data Systems by Comparing with the Other Four Systems (ACR TI-RADS, Kwak-TIRADS, KSThR-TIRADS, and EU-TIRADS): a Single-Center Study. Front Endocrinol. 2021;12:763897. doi:10.3389/fendo.2021.763897
23. Kang YJ, Stybayeya G, Lee JE, et al. Diagnostic performance of ACR and Kwak TI-RADS for benign and malignant thyroid nodules: an update systematic review and meta-analysis. Cancers. 2022;14(23):5961. doi:10.3390/cancers14235961
24. Hu Y, Xu S, Zhan W. Diagnostic performance of C-TIRADS in malignancy risk stratification of thyroid nodules: a systematic review and meta-analysis. Front Endocrinol. 2022;13:938–961. doi:10.3389/fendo.2022.938961
25. Eccher A, Girolami I, D’Errico A, et al. Management of thyroid nodules in deceased donors with comparison between fine needle aspiration and intraoperative frozen section in the setting of transplantation. Prog Transplant. 2019;29:316–320. doi:10.1177/1526924819873898
26. Girolami I, Marletta S, Pantanowitz L, et al. Impact of image analysis and artificial intelligence in thyroid pathology, with particular reference to cytological aspects. Cytopathology. 2020;31(5):432–444. doi:10.1111/cyt.12828
27. Marletta S, Salatiello M, Pantanowitz L, et al. Delphi expert consensus for whole slide imaging in thyroid cytopathology. Cytopathology. 2023. Doi:10.1111/cyt.13279