Health Care, Insight
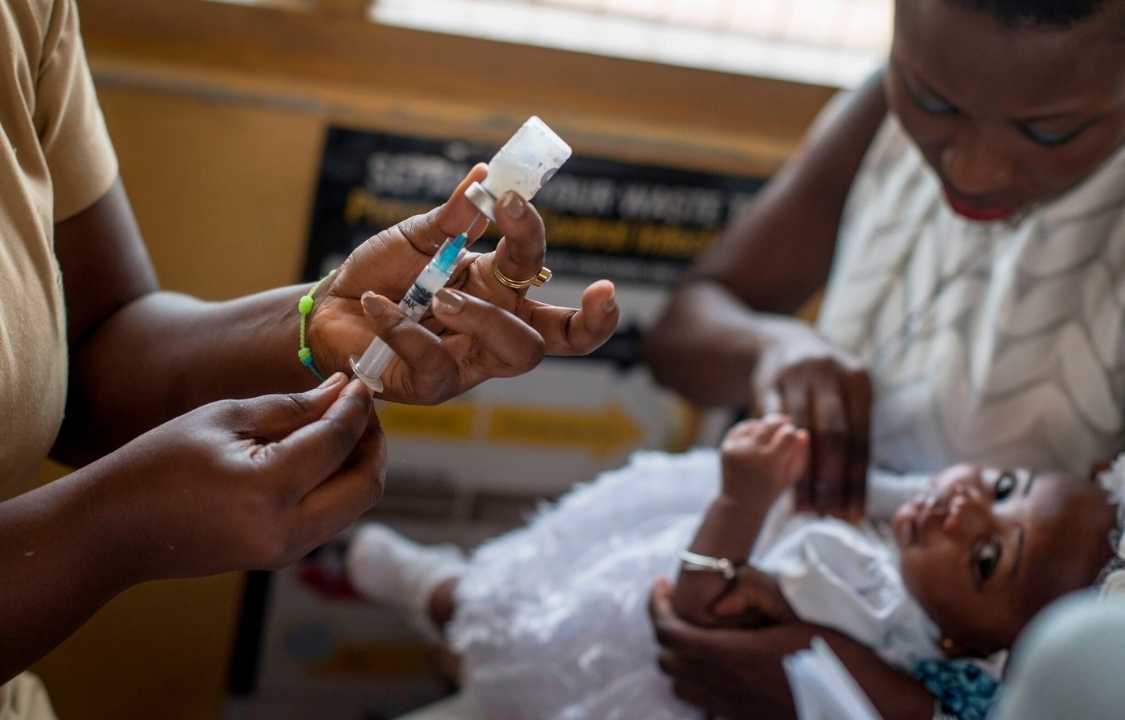
First Malaria Vaccine Approved by WHO: ‘This Is a Historic Moment’
The vaccine is the first of its kind—not only for malaria, but for parasitic diseases in general.
The World Health Organization (WHO) recently made an historic announcement that has the potential to transform global health: the endorsement of a novel malaria vaccine, RTS,S (Mosquirix). This groundbreaking vaccine, developed by GlaxoSmithKline (GSK), specifically targets Plasmodium falciparum, the deadliest malaria parasite globally. Malaria, a mosquito-borne disease, continues to claim countless lives, with sub-Saharan Africa bearing the heaviest burden. In this comprehensive article, we delve into the significance of this historic development, the impact of the vaccine, its potential implications for global health, and what lies ahead in the fight against malaria.
Understanding Malaria: A Global Health Challenge
Malaria is a formidable global health challenge, affecting millions of lives each year. In 2019 alone, malaria claimed an estimated 409,000 lives, with a majority of deaths occurring in sub-Saharan Africa. Tragically, children under the age of 5 accounted for 274,000 of these deaths, underscoring their vulnerability to this disease. While malaria cases in the United States are relatively rare, it remains a pervasive threat in less developed nations, making it a top priority in global health initiatives.
The Quest for an Effective Malaria Vaccine
For centuries, malaria has plagued humanity, inflicting immense suffering and death. Numerous attempts to develop an effective malaria vaccine have been met with disappointment. Malaria is a particularly challenging target due to the complexity of parasitic diseases, which differ significantly from bacterial and viral diseases. However, the recent endorsement of the RTS,S malaria vaccine represents a historic breakthrough in the battle against this ancient scourge.
Key Insights into the New Malaria Vaccine
1. Target Population: The WHO recommends the RTS,S malaria vaccine for children residing in regions with moderate to high malaria transmission, as defined by WHO. This primarily encompasses sub-Saharan Africa, where the majority of malaria cases and fatalities occur. However, the WHO also identifies at-risk regions in South-East Asia, Eastern Mediterranean, Western Pacific, and the Americas.
2. Age Group: The vaccine is intended for children starting at 5 months of age. The recommendation is based on data from pilot programs conducted in Ghana, Kenya, and Malawi over the past two years.
3. Dosage: The RTS,S vaccine is administered in four separate doses. The vaccine’s effectiveness was observed when it was administered in conjunction with other preventive interventions, such as sleeping under insecticide-treated bednets.
4. Impact: The pilot programs in Ghana, Kenya, and Malawi yielded promising results, demonstrating a 30% reduction in severe malaria cases among vaccinated children. When combined with bednet usage, over 90% of children experienced protection. Importantly, the vaccine did not negatively impact the use of other preventive measures.
5. Efficacy Over Time: While the vaccine’s efficacy declined over time, a booster dose significantly enhanced protection. The ideal scenario involved three primary doses administered over three months, followed by a booster dose 18 months later.
The Path Forward for the Malaria Vaccine
The WHO’s endorsement of the RTS,S malaria vaccine represents a significant step forward, but several critical steps lie ahead:
1. Funding and Global Support: Broader deployment of the vaccine hinges on funding decisions from the global health community. Gavi, the global vaccine alliance, along with other stakeholders, will play a crucial role in determining the financing and implementation of the vaccine in sub-Saharan African countries.
2. Continued Research: Ongoing research will focus on the vaccine’s long-term effectiveness, especially in relation to the booster dose. Researchers will also assess how immunization impacts child mortality due to malaria.
3. Improvements and Modifications: While the vaccine is a milestone, further refinements are necessary to enhance its efficacy. Researchers and scientists will continue to work on optimizing the vaccine.
4. Global Health Cooperation: International collaboration and cooperation will be vital in the fight against malaria. The malaria vaccine serves as a testament to what can be achieved when public and private entities work together.
Conclusion: A Historic Stride Towards Malaria Eradication
The endorsement of the RTS,S malaria vaccine by the WHO marks an unprecedented advancement in global health. After decades of research and development, this vaccine offers hope to regions burdened by the devastating impact of malaria. While challenges and questions remain, this momentous achievement underscores the potential for scientific innovation to transform the trajectory of deadly diseases.
The road ahead is long, but the global health community, united in purpose, can forge a path towards malaria eradication. The historic significance of this vaccine cannot be overstated—it represents a glimmer of hope for a world free from the specter of malaria, a goal that has eluded humanity for centuries. As WHO Director-General Dr. Tedros Adhanom Ghebreyesus aptly stated, “We still have a very long road to travel, but this is a long stride down that road.”
The development and endorsement of the RTS,S vaccine signify a culmination of tireless efforts from scientists, healthcare workers, governments, and organizations worldwide. It exemplifies what can be achieved through international collaboration and investment in research and development. However, the vaccine’s implementation presents its own set of challenges, including ensuring equitable access, distribution, and addressing potential logistical hurdles.
Equity in vaccine access is paramount. It is imperative that the benefits of this breakthrough are shared globally, particularly with the most vulnerable populations in malaria-endemic regions. Steps must be taken to ensure that cost and logistical constraints do not hinder access to the vaccine for those who need it most.
Additionally, a robust monitoring and evaluation system should be put in place to track the vaccine’s effectiveness and safety in real-world settings. This data will be crucial for making any necessary adjustments and optimizing its impact on reducing malaria cases and deaths.
Furthermore, the endorsement of the RTS,S vaccine should serve as a reminder of the importance of continued investment in research and development for global health. Diseases like malaria remain formidable challenges, but with dedication, innovation, and international cooperation, we can take significant strides towards their eradication.
In conclusion, the endorsement of the RTS,S malaria vaccine represents a historic milestone in global health. While challenges persist, the global community must remain committed to ensuring equitable access, monitoring its impact, and continuing the pursuit of a malaria-free world. This vaccine offers a beacon of hope and serves as a testament to the power of scientific innovation and international collaboration in the fight against deadly diseases.