Cancer and neoplasms
Biologic Treatment did not Significantly Increase Hematologic Malignancy Risk in PsA, PsO
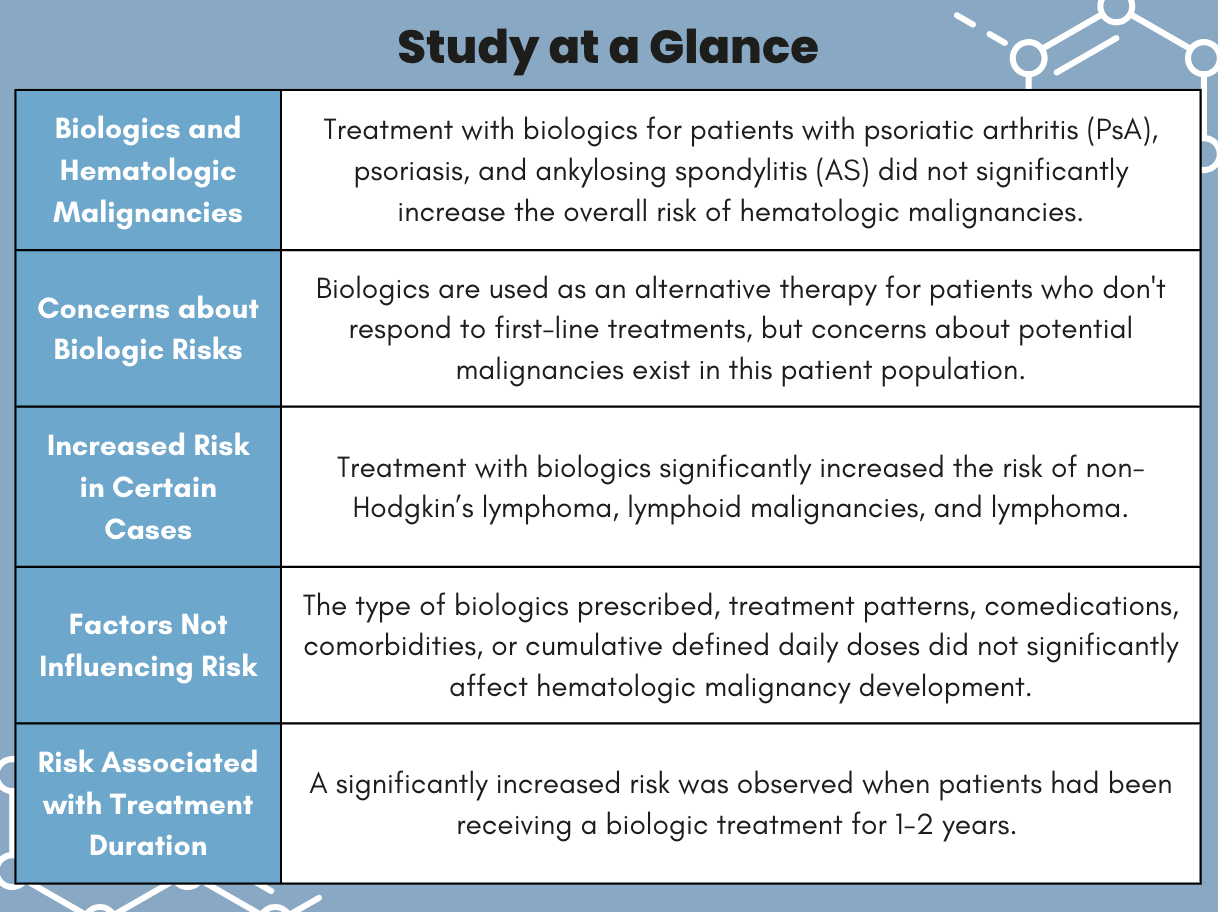
In patients with psoriatic arthritis (PsA), psoriasis, and ankylosing spondylitis (AS), treatment with biologics did not significantly increase the overall risk of hematologic malignancies. However, investigators observed a significant increased risk in those treated with biologics between 1 – 2 years and for developing certain types of lymphoma, according to research published in Biomedicines.1
Although autoimmune diseases have been previously linked to hematologic malignancies, studies have reported inconsistent results regarding biologic treatment and the development of malignancies in this patient population. This classification of drug is often prescribed as an alternative therapy for patients who had inadequate or non-response to first-line therapy, which often includes non-biologics such as glucocorticoids, nonsteroidal anti-inflammatory drugs (NSAIDs), and conventional synthetic disease-modifying antirheumatic drugs (csDMARDs). While biologics have been proven to improve disease activity and increase quality of life, there are concerns about the risks of comorbidities, including malignancies, in this patient population.2
“Not only are the risks of hematologic malignancy with biologics use disputed but also the data on patients with AS and PsA are scarce,” wrote co-lead investigator Yu-Chih Lin, PhD, associated with the School of Medicine and Department of Internal Medicine at Kaohsiung Medical University in Taiwan, and colleagues. “Because rheumatoid arthritis (RA) is more prevalent than other autoimmune diseases, most studies have focused on the effect of biologics (eg, tumor necrosis factor inhibitors (TNF) on patients with RA.”
Data from Taiwan’s National Health Insurance Research Database (NHIRD) between 2010 and 2020 were used to evaluate hematologic malignancy risk in this retrospective cohort study. Patients with psoriasis, PsA, and AS were placed in either the biologic or non-biologic cohorts after 1:10 propensity score matching (PSM). Poisson regression analyzed the incidence rate ratio (IRR) of the hematologic malignancy incidences and the time-/dose-dependent effects on biologics.
Hematologic malignancies were categorized as either lymphoid (lymphoma, lymphoid leukemia, multiple myeloma, immunoproliferative neoplasms, and other malignant tumors of the lymphatic tissue) or myeloid (leukemia, monocytic leukemia, and other specified leukemias).
A total of 4157 patients were included in the biologics cohort and 38,399 were in the non-biologic group. The mean age at index date was 45.3 years, and most (65%) were male. Of these patients, 10 patients developed hematologic malignancies in the biologics group and 72 developed malignancies in the non-biologics cohort. In general, biologic medication did not increase the risk of hematologic cancers in this patient population.
However, treatment with biologics significantly increased the risk of these malignancies in non-Hodgkin’s lymphoma (IRR: 2.48, 95% confidence interval [CI]: 1.28 – 4.80, P = .01), lymphoid malignancies (IRR: 1.78, 95% CI: 1.08 – 2.93, P = .02), and lymphoma (IRR: 2.78, 95% CI: 1.50 – 5.18, P <.01).
Hematologic malignancy was not affected by the type of biologics prescribed, treatment patterns, comedications, comorbidities, or cumulative defined daily doses. However, the risk of developing these malignancies was significantly increased when a patient had been receiving a biologic between 1 – 2 years (IRR: 2.95, 95% CI: 1.14 – 7.67). Interestingly, no increased risk was observed in patients receiving biologics for <1 year or ≥2 years.
Investigators noted the NHIRD database does not contain information on disease severity, laboratory results, smoking status, or a patient’s personal or family history. To account for this, patients were matched and adjusted for disease duration and comedication usage. Strengths of the study included the large sample size designed to better identify the effects of biologics on hematologic cancer development in this patient population. Additionally, all patients included in the analysis had received treatment for ≥3 months. The strict inclusion criteria was implemented to clarify whether malignancies were associated with the treatment or with the underlying autoimmune disease. Lastly, the subtypes of hematologic malignancies and biologics dose-response effects were assessed in detail.
“These findings should alert healthcare professionals to the possible impact of biologics on the development of hematologic malignancies in patients with AS, psoriasis, and PsA and encourage further research into the mechanisms involved,” investigators concluded.
References
- Tsai CJ, Lin YC, Chen CY, Hung CH, Lin YC. The Effects of Biologics on Hematologic Malignancy Development in Patients with Ankylosing Spondylitis, Psoriasis, or Psoriatic Arthritis: A National Cohort Study. Biomedicines. 2023;11(9):2510. Published 2023 Sep 11. doi:10.3390/biomedicines11092510
- Lopez-Olivo, M.A.; Tayar, J.H.; Martinez-Lopez, J.A.; Pollono, E.N.; Cueto, J.P.; Gonzales-Crespo, M.R.; Fulto, S.; Suarez-Almazor, M.E. Risk of malignancies in patients with rheumatoid arthritis treated with biologic therapy: A meta-analysis. JAMA 2012, 308, 898–908