Cancer and neoplasms
Retrospective Analysis of Ocular Cancer in Saudi Arabia
Introduction
Ocular oncology holds a distinctive role within the field of ophthalmology. Not only do these conditions pose a significant threat to vision, but they also carry the risk of potential eye loss, and some of them can even impact life expectancy.1 The development of specific tumor care procedures and the establishment of policies are heavily relied on registry data. The ophthalmic literature has a large amount of information on particular malignant periocular and ocular neoplasms from various countries.2
Studies on the epidemiology of ophthalmologic malignancies have been published in many countries with pathology registries. Clinicians find this information to be very helpful in identifying and treating their patients. It should be noted that significant differences exist in the incidence and nature of ocular malignancies between various population groups. Compared to Asian countries, western countries usually have higher incidence rates of ocular cancer.3 In the United States, for example, the yearly incidence rate is roughly 1 per 100,000, while the prevalence is approximately 12 per 100,000.4 In contrast, the yearly age-standardized incidence of ocular cancer is lower in countries such as Shanghai, Taiwan, Singapore, and Japan, ranging from 1.8 to 3 persons per million population.5 Melanomas are recognized as the most common primary intraocular malignancies among Caucasians but are uncommon in other ethnicities, are a perfect example of this.6 Retinoblastomas are substantially more common than uveal melanomas in Asian countries such as Singapore, accounting for more than half of all reported cases of eye cancer.3 Non-pigmented eyelid tumors, such as basal cell carcinomas (BCC), squamous cell carcinomas (SCC), and sebaceous gland carcinomas (SGC), are significantly more common in the area than malignant melanomas.7 Conjunctival tumors include a wide spectrum of lesions that can arise from a variety of cell types.8 Several variables, including ethnicity and geography, have been found to be associated with the incidence of epithelial and melanocytic lesions. Notably, in Africa, the co-infection of human immunodeficiency virus (HIV) and human papillomavirus (HPV) has been related to an increase in the frequency of ocular surface squamous neoplasia, particularly in women.9
In the Kingdom of Saudi Arabia (KSA), a total of 27,885 cancer cases were identified in 2020. 14,253 of these cases involved men, while 13,632 involved women. Unfortunately, cancer was responsible for 13,069 deaths during that time. In KSA, the general probability of acquiring cancer before the age of 75 is 9.9%. For men, the risk is 9.1% and for women, it is 11.4%. Breast cancer, colorectal cancer (CRC), prostate cancer, brain/central nervous system cancer, Hodgkin and non-Hodgkin lymphoma, kidney cancer, and thyroid cancer are the most common cancers in KSA.10 The epidemiology of ocular malignancies in Saudi Arabia is still unclear. Therefore, the main objective of this study was to use histological diagnoses to determine the most common types of eye and ocular adnexa malignancies across different ages and sex.
Materials and Methods
Access to the tumor registry data from 1994 to 2018 was permitted by King Khaled University’s institutional review board. Research followed the guidelines set forth in the Helsinki Declaration. The data evaluation was carried out by an expert team. All cases of ocular malignancy that were documented and verified by histopathological reports during the course of the research period were included in the current analysis. The Ophthalmic Oncology team received referrals from various places within the KSA for the diagnosis and treatment of patients with ocular and adnexal neoplasms. Tumor biopsies, lesion excisions, enucleations, or exonerations were carried out for diagnostic and therapeutic purposes, and the samples were then forwarded to the Ophthalmic Pathology division to make the final diagnosis decision. The registry recorded patient data, such as demographics like age (Age groups are organized into five-year intervals, starting from under 5 years old and extending to over 75 years old, with each interval spanning an equal range of years.), sex, and nationality, clinical information, and tumor classification. The affected eye, the origin of the ocular tissue, and the clinical diagnosis were all noted as clinical characteristics. The final diagnosis was recorded using the International Classification of Diseases, Tenth Revision and Australian Modification (ICD-10 AM) information. To maintain the confidentiality of patients for this investigation, the data were deidentified. The Statistical Package for Social Sciences version 26 (SPSS Inc, Chicago, IL, USA) was used to analyze the tumor registry data from 1994 to 2018 imported from a spreadsheet. The total number of cases identified during this time was summed. We divided the total number of cases diagnosed within each region by the total number of cases reported in an attempt to describe the number of cases diagnosed within each region. Finally, we calculated the proportion of cases among nationalities by dividing the total number of cases of ocular cancer into Saudi and non-Saudi cases. To report the proportion of cases diagnosed within each age group, we divided the number of cases diagnosed within each age category by the overall number of cases diagnosed. We used percentages to derive frequencies for qualitative variables. In order to display the prevalence of cases across the several Saudi Arabian provinces (Riyadh, Makkah, Eastern, Medina, Al Baha, Al Jawf, Northern Borders, Qassim, Ha’il, Tabuk, Aseer, Jizan, and Najran), we created a heat map in Excel. We used Excel’s graph tool to make a timeline that shows the frequency of new cases of ocular cancer in both sexes during the study period.
Results
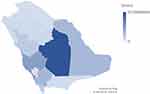
The total number of cases with ocular cancer diagnosed was 1056 cases. Figure 1 presents the incidence of ocular cancer in different regions in Saudi Arabia. The data indicates varying number of ocular cancer cases across the regions. Riyadh has the highest number of reported cases at 35.39% (n = 372), followed by Makkah at 16.93% (n = 178). The Eastern region and Aseer also show relatively higher rates at 10.47% and 10.94%, respectively. On the other hand, regions like Medina, Al Baha, Al Jawf, Northern Borders, Qassim, Ha’il, Tabuk, Jizan, and Najran have lower incidence rates ranging from 1.43% to 6.18%.
|
Figure 1 The geospatial distribution of ocular cancer in Kingdom of Saudi Arabia from 1998 to 2018 across different regions. |
Figure 2 displays the proportion of ocular cancer based on different age groups. The data indicates variations in the occurrence of ocular cancer across different age categories. The highest proportion was observed in the 0–4 age group (55.47%, n = 583). There is a notable decline in with increasing age, with lower rates seen in older age groups. The 75+ age group shows the second-highest proportion of cases (9.80%, n = 103). Age groups between 60–64 years, 70–74 years, and 55–59 years also exhibit relatively higher rates, at 6.82%, 5.02%, and 2.65%, respectively.
 |
Figure 2 The proportion of cases diagnosed with of ocular cancer of different age category from 1998 to 2018 in Kingdom of Saudi Arabia. [Age groups are organized into five-year intervals, starting from under 5 years old and extending to over 75 years old, with each interval spanning an equal range of years]. |
Figure 3a presents the annual number of reported eye cancer cases from 1994 to 2018, indicating fluctuations in the number of cases over the 25-year period. While there are certain years with higher recorded cases, like 1997, 2009, and 2018, with 60 cases each, there are also years with lower numbers, such as 1995 and 2014, with 33 and 43 cases, respectively. The Figure 3b presents the percentage distribution of males and females among reported eye cancer cases for each year from 1994 to 2018. The data reveals variations in gender representation over the 25-year period. In some years, there were higher percentages of males affected by eye cancer, while in other years, females had a higher representation. For instance, in 1994 and 2018, the percentage of males was significantly higher at 64.00% and 67.24%, respectively. Conversely, in 1995 and 2014, females constituted a higher percentage at 57.58% and 62.79%, respectively. Figure 3c presents the percentage distribution of Saudi and Non-Saudi individuals among reported ocular cancer cases for each year from 1997 to 2018. The data reveals variations in the representation of both groups over the 22-year period. In some years, a higher percentage of Saudi individuals were affected by eye cancer, while in other years, non-Saudi individuals had a high representation. For instance, in 1999, the percentage of Saudi individuals was significantly higher at 90.57%, while in 2003, the non-Saudi representation was notably high at 41.67%.
|
Figure 3 (a) Number of cases with ocular cancer in Kingdom of Saudi Arabia, (b) percentage of cases across both sexes, (b) percentage of ocular cancer among Saudi and non-Saudi residents. |
Figure 4 depicts the crude incidence of ocular cancer among males and females for each year from 1994 to 2018. The data indicates low incidence rates for both genders, with most of the reported rates being below 1%. The incidence among males and females generally remains relatively stable over the years, with slight variations observed between 0.1% to 0.5%. While there are some slight differences in incidence between males and females, both genders exhibit similar patterns over time.
|
Figure 4 The incidence of ocular cancer from 1998 to 2018 across time in Kingdom of Saudi Arabia. |
Figure 5 presents the percentage distribution of different ocular cancer pathologies. The data reveals the prevalence of various types of ocular malignancy. The overall presentation of retinoblastoma was 92.04 categorized as follow, “Retinoblastoma, NOS”, (56.32%), “Retinoblastoma, undifferentiated” (25.13%), and “Retinoblastoma, diffuse” (7.93%). And Retinoblastoma, differentiated (2.70%). Additionally, “Embryonal rhabdomyosarcoma, NOS” was less prevalent, comprising 2.53%. The remaining pathologies have lower percentages, ranging from 0.17% to 1.85%.
|
Figure 5 The histopathological diagnosis of ocular cancer cases in Saudi Arabia from 1997 to 2018. |
Discussion
Many studies have been conducted worldwide to describe the pattern of ocular tumor at national level11,12 or at local level.13,14 This research has succeeded in mapping of ocular cancer in the studied countries in term of incidence, gender differences, pathology, and natural history. In this research we conducted an examination of a substantial group of patients affected by ocular neoplasms within the Saudi population. The data from the registry covers a period of 25 years, allowing for the observation of trends in ophthalmic malignancies over time. The national cancer registry for the Kingdom of Saudi Arabia was founded in 1999, while records of particular information about ocular malignancies date back to 1983. The study revealed varying number of across regions, with Riyadh having the highest number of cases at 35.39%, followed by Makkah at 17.03%. Age-specific incidence indicated a higher occurrence in the 0–4 age group at 55.21%, decreasing with age. The data also showed fluctuations in reported cases over the years, with variations in gender and nationality representation among eye cancer cases. The incidence rates among males and females remained relatively stable over time, while different ocular cancer pathologies were observed, with “Retinoblastoma, NOS” being the most prevalent.
Incidence of Ocular Cancer
Ocular malignancies are considered rare and there is limited knowledge about their distribution and underlying factors. Timely diagnosis and treatment of ocular malignancies are important because they endanger both vision and life.15 Based on the World Health organization in 2020, in total 27,855 cases of malignancy were diagnosed with 13,069 deaths among nearly 35 million Saudi citizens. Breast, thyroid, colon and rectum cancer ranked on the top of the list with nearly 10 thousand cases reported in 2020.16 In this study, incidence of ocular cancer among Saudi population ranged from 0.2 to 0.3 per 100,000 population except for 2018 when the incidence among males peaked to 2.2 per 100,000. Akiki et al,15 used information from the Lebanese National Cancer Registry to examine case of eye cancer in Lebanon from 2005 to 2016. The incidence rate was 0.24 per 100,000 in men and 0.22 per 100,000 in women. The average age of ocular cancer patients was, however, considerably older than our findings, at 31.94 years for men and 22.04 years for women. According to the Cancer Incidence in Five Continents study conducted in 2014, the worldwide annual incidence of ocular malignancies varies from 0.1 to 7.4 cases per 100,000 individuals. In general, western countries have greater incidence rates than Asian countries do.3 For instance, in the United States, the annual incidence rate of ocular cancer is approximately 1 case per 100,000 individuals, with a prevalence of 12 cases per 100,000 individuals.15 On the other hand, countries like Singapore, Taiwan, Shanghai, and Japan have lower annual age-standardized incidence rates, ranging from 1.8 to 3 cases per million population.3,5 The differences in incidence rates between regions highlight the need for further research to better understand the contributing factors to ocular malignancies.
We found that incidence of ocular cancer among males was relatively higher than females across most of the study period. Many studies reported similar findings in Taiwan,5 Singapore,3 and Iran.17 Men have a higher prevalence due to their propensity for more outdoor sun exposure.18 However, this sex pattern was not observed in a study that comparing uveal melanoma (UM) cases between men and women. The study did not find significant gender differences in symptoms, age at diagnosis, tumor size, or recurrence rate. However, men were more likely to have tumors located posterior to the equator and suffered more metastases. The time until metastases development was shorter in men, and the cumulative incidence of melanoma-related mortality was higher in men.19
We discovered that the incidence of eye cancer had two separate peaks, or a bimodal distribution. A relatively high incidence of eye cancer in young children is indicated by the first peak, which is seen in the age range of 0–4 years. The second peak occurs among those above the age of 75 years, indicating another higher incidence of eye cancer in older individuals. In addition to the two clusters observed, the study identified an additional cluster of higher eye cancer between the ages of 60 and 64 years. Similar age peaks were observed among Lebanese. Akiki et al,15 found that age-specific rates of eye cancer in males follow a bimodal distribution, with two distinct clusters of higher incidences. The first cluster is seen in children between the ages of 0 and 4 years. Males over 50 years old exhibit the second cluster. Furthermore, Shields et al20 performed a systematic evaluation of 1,264 individuals over a 30-year period and discovered that benign lesions were more prevalent than malignant lesions, but that malignancy increased with age. Children (age 0–18) had 20% of malignant lesions, followed by young adults and middle-aged patients (ages 19–59), with a percentage of 27%, and older patients (age range, 60+), with a percentage of 58%. The most frequent cancer in older people was lymphoma, which accounted for 10% of cases. The most common cancer in young people was rhabdomyosarcoma, which accounted for 3% of all orbital masses. Similar results were found in studies conducted in Japan21 and India,22 indicating that the origins and incidence rates of children’s orbital cancers differ from those of adult tumors.
Ocular Cancer Pathology
In this study the most frequently diagnosed ocular cancer was retinoblastoma. Retinoblastoma is an uncommon childhood eye tumor that develops in the retina. Mutations in the RB1 gene are the main cause of retinoblastoma cases. The most common intraocular cancer in children is retinoblastoma, with reported incidence rates of 1 in 15,000 to 1 in 18,000 live births. Regarding the frequency of incidence among malignant intraocular malignancy, it is only surpassed by uveal melanoma. Furthermore, there is no evidence of a racial or gender predisposition in retinoblastoma occurrence.23 Leukocoria and strabismus are the two most common signs of retinoblastoma. There may also be iris rubeosis, hypopyon, hyphema, buphthalmia, orbital cellulites, and exophthalmia. Sixty percent of retinoblastomas are unilateral, and the majority of these types are not inherited (median age at diagnosis two years). In 40% of cases, retinoblastoma is bilateral (median age at diagnosis one year). Hereditary are all bilateral and multifocal unilateral types.24 Huaman et al,25 presented the baseline for the geographic incidence of eye tumors in Saudi Arabia between 1982 and 1989. The most prevalent malignant tumors are retinoblastoma, SCC of the conjunctiva, basal cell carcinoma of the eyelid, and malignant melanoma, listed in decreasing frequency. Similarly, the most frequent benign tumors are nevi, epithelial cysts, hemangiomas, and dermoid cysts, in that order. Based on information from the King Khaled Eye Specialist Hospital’s tumor registry and statistics from the Saudi population, Khandekar et al26 offered an epidemiologic profile and the incidence of ocular malignant tumors. They discovered that retinoblastoma, which accounts for 91% of cases, was the most prevalent ocular cancer in youngsters. SCC accounted for 45.8% of instances of MT in adults, followed by BCC (23%), uveal melanoma (11.9%), sebaceous gland carcinoma (6.8%), lymphomas (orbital, adnexal) (5.8%), and others (6.8%). Similarly, according to statistics from the cancer registry among the Chinese population, retinoblastoma—which accounts for 35.3% of cases—was the most prevalent malignancy, followed by melanoma (17.9%) and lymphoma (13.8%).5 On the other hand, retinoblastoma made up 7.1% of the 210 pathology reports of orbito-ocular biopsies, while SCC made up the bulk, including 82.1% of cases.27 The predominance of squamous cell carcinoma in the other studies could be attributed to the overexposure to excessive sunlight in Africa. This is not the cases among Saudi, due to cultural and religious issue females cover their body and face reducing the body area exposed to the sun.
Strengths and Limitations
Due to the distinctive ocular oncology practices and referral patterns across the country, the incidence of ocular malignant neoplasms generated from this hospital-based registry gives a trustworthy estimate for the whole Kingdom of Saudi Arabia. However, rare cases of malignant tumors that were unsuitable for treatment and were sent to a general hospital after the initial assessment were probably the only ones that were not recorded in the register.
Conclusions
Ocular cancer incidences in Saudi Arabia have drastically varied throughout the country’s various regions and age categories from 1998 to 2018, according to data on the disease. The highest incidence is observed in Riyadh, followed by Makkah, while certain regions have lower rates. Additionally, the incidence of ocular cancer varies among different age groups, with the highest occurrence in the 0–4 age group. The study also highlights fluctuations in the annual number of reported cases, suggesting potential influences from various factors. Furthermore, there are variations in the gender and nationality distribution among reported cases over the years. Overall, the study emphasizes the need for continued monitoring, research, and analysis of potential factors influencing ocular cancer occurrence in Saudi Arabia. Understanding the patterns and prevalence of ocular cancer in different regions and age groups can help healthcare authorities and policymakers devise targeted strategies for prevention, early detection, and treatment of this condition.
Data Sharing Statement
Data available upon request by emailing the corresponding author.
Ethical Approval
The King Khaled University’s institutional review board provided ethical approval. All respondents provided written consent in accordance with IRB standards based on local laws. The study followed the guidelines set forth in the Helsinki Declaration.
Consent for Publication
The study was approved by the Ethical committee of the King Khalid University [IRB: ECM#2023-2304].
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research received no external funding.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Chadha V, Sagoo MS. Ocular oncology demystified. Eye. 2023;37(5):795–796. doi:10.1038/s41433-022-02343-5
2. Shields CL, Demirci H, Karatza E, et al. Clinical survey of 1643 melanocytic and nonmelanocytic conjunctival tumors. Ophthalmology. 2004;111(9):1747–1754. doi:10.1016/j.ophtha.2004.02.013
3. Lee SB, Eong KG, Saw SM, et al. Eye cancer incidence in Singapore. Br J Ophthalmol. 2000;84(7):767. doi:10.1136/bjo.84.7.767
4. Kleinstein RN, Lehman HF. Incidence and prevalence of eye cancer. Optomet Vision Sci. 1977;54(1):49–51. doi:10.1097/00006324-197701000-00008
5. Cheng CY, Hsu WM. Incidence of eye cancer in Taiwan: an 18-year review. Eye. 2004;18(2):152–158. doi:10.1038/sj.eye.6700619
6. Jovanovic P, Mihajlovic M, Djordjevic-Jocic J, et al. Ocular melanoma: an overview of the current status. Int J Clin Exp Pathol. 2013;6(7):1230.
7. He J, Liu D, Zhuang J, Wu H, Lin S. Eyelid tumours and pseudotumours in Hong Kong: a ten-year experience. Hong Kong Med J. 2013;19(2):150–155.
8. Saornil M, Becerra E, Méndez MC, et al. Conjunctival tumors. Arch Soc Esp Oftalmol. 2009;84(1):7–22. doi:10.4321/s0365-66912009000100003
9. Gichuhi S, Sagoo MS, Weiss HA, et al. Epidemiology of ocular surface squamous neoplasia in A frica. Tropical Med Inter Health. 2013;18(12):1424–1443. doi:10.1111/tmi.12203
10. Bahamdan KA, Morad NA. Pattern of malignant skin tumors in Asir region, Saudi Arabia. Ann Saudi Med. 1993;13(5):402–406. doi:10.5144/0256-4947.1993.402
11. Martel A, Nahon-Esteve S, Gastaud L, et al. Incidence of orbital exenteration: a nationwide study in France over the 2006–2017 period. Ophthalmic Epidemiol. 2021;28(2):169–174. doi:10.1080/09286586.2020.1795887
12. Jung S-K, Lim J, Yang S-W, et al. Nationwide trends in the incidence and survival of eyelid skin cancers in Korea. Ophthalmic Epidemiol. 2020;27(6):438–448. doi:10.1080/09286586.2020.1767152
13. Mirzayev I, Gündüz AK, Gündüz ÖÖ, et al. Demographic and clinical features of conjunctival tumours at a tertiary care centre. Clin Experl Optom. 2022;105(7):708–714. doi:10.1080/08164622.2021.1971048
14. Eren MA, Gündüz AK. Demographic features and histopathological diagnosis in primary eyelid tumors: results over 19 years from a tertiary center in Ankara, Turkey. Int J Ophthalmol. 2020;13(8):1287. doi:10.18240/ijo.2020.08.16
15. Akiki D, El Hage S, El Masri J, et al. Epidemiology of ocular malignancies among the Lebanese population: a 12-year review. Cureus. 2022;14(1):e21593. doi:10.7759/cureus.21593
16. World Health Organization. Cancer Today. Saudi Arabia; 2020. Available from: https://gco.iarc.fr/today/data/factsheets/populations/682-saudi-arabia-fact-sheets.pdf. Accessed October 11, 2023.
17. Ahmadi SA, Asadi AF, Gouhari MK. Ocular tumors in Iran: a 10-year histopathological study on 384 cases of enucleation; 2008.
18. Vajdic CM, Kricker A, Giblin M, et al. Incidence of ocular melanoma in Australia from 1990 to 1998. Inter J Cancer. 2003;105(1):117–122. doi:10.1002/ijc.11057
19. Zloto O, Pe’er J, Frenkel S. Gender differences in clinical presentation and prognosis of uveal melanoma. Invest Ophthalmol Vis Sci. 2013;54(1):652–656. doi:10.1167/iovs.12-10365
20. Shields JA, Shields CL, Scartozzi R. Survey of 1264 patients with orbital tumors and simulating lesions: the 2002 Montgomery Lecture, part 1. Ophthalmology. 2004;111(5):997–1008. doi:10.1016/j.ophtha.2003.01.002
21. Ohtsuka K, Hashimoto M, Suzuki Y. A review of 244 orbital tumors in Japanese patients during a 21-year period: origins and locations. Jpn J Ophthalmol. 2005;49:49–55. doi:10.1007/s10384-004-0147-y
22. Bajaj MS, Pushker N, Amrita Chaturvedi MD, et al. Orbital space-occupying lesions in Indian children. J Pediatr Ophthalmol Strabismus. 2007;44(2):106.
23. Ancona-Lezama D, Dalvin LA, Shields CL. Modern treatment of retinoblastoma: a 2020 review. Indian J Ophthalmol. 2020;68(11):2356–2365. doi:10.4103/ijo.IJO_721_20
24. Aerts I, Lumbroso-Le Rouic L, Gauthier-Villars M, et al. Retinoblastoma. Orphanet J Rare Dis. 2006;1:31. doi:10.1186/1750-1172-1-31
25. Huaman A, Cavender JC. Tumors of the eye in Saudi Arabia. Ann Saudi Med. 1991;11(6):675–680. doi:10.5144/0256-4947.1991.675
26. Khandekar RB, Al-Towerki AA, Al-Katan H, et al. Ocular malignant tumors. Review of the Tumor Registry at a tertiary eye hospital in central Saudi Arabia. Saudi Med J. 2014;35(4):377–384.
27. Sinyiza FW, Chisale MRO, Kayira AB, et al. Histopathological profile of orbito-ocular cancers at a tertiary hospital in Northern Malawi: a retrospective cross-sectional study. BMJ Open Ophthalmol. 2022;7(1):e000977. doi:10.1136/bmjophth-2022-000977