Cardiovascular
Philips Showcases Latest Medical Innovations and Solutions for Patients with Cardiovascular Disease TCT23
October 20, 2023 — Over the coming days, Philips will be presenting its latest solutions in cardiology and new late-breaking clinical data at the Transcatheter Cardiovascular Therapeutics (TCT) annual meeting (October 23-26 in San Francisco, USA). Philips will feature a comprehensive portfolio of image-guided therapy and echocardiography solutions that integrate advanced imaging systems and software with specialized diagnostic and therapeutic devices, creating a holistic approach to support high-quality treatment, even for complex medical procedures. The company will also share clinical data and cutting-edge research from a five-year study comparing patient outcomes in the diagnosis and treatment of heart disease using instant wave-free ratio (iFR) and fractional flow reserve (FFR).
At TCT, the Philips Engagement Hub, located in the exhibit hall at the Moscone Center, will offer a well-rounded variety of activities, including physician-led discussions, in-depth symposia, and hands-on training sessions. These events are designed to address a range of key challenges in the field of cardiology, providing valuable opportunities for knowledge exchange and professional development.
“As a leader in cardiology innovation, Philips is uniquely positioned to deliver solutions that integrate imaging, devices, software, informatics and services at each point of a heart patient’s journey,” said Bert van Meurs, Chief Business Leader, Image Guided Therapy and Precision Diagnosis. “By collaborating with our customers to bring purposeful innovation to cardiology and beyond, we further validate our advanced technologies and the clinical and economic evidence supporting them – all to improve patient care more efficiently.”
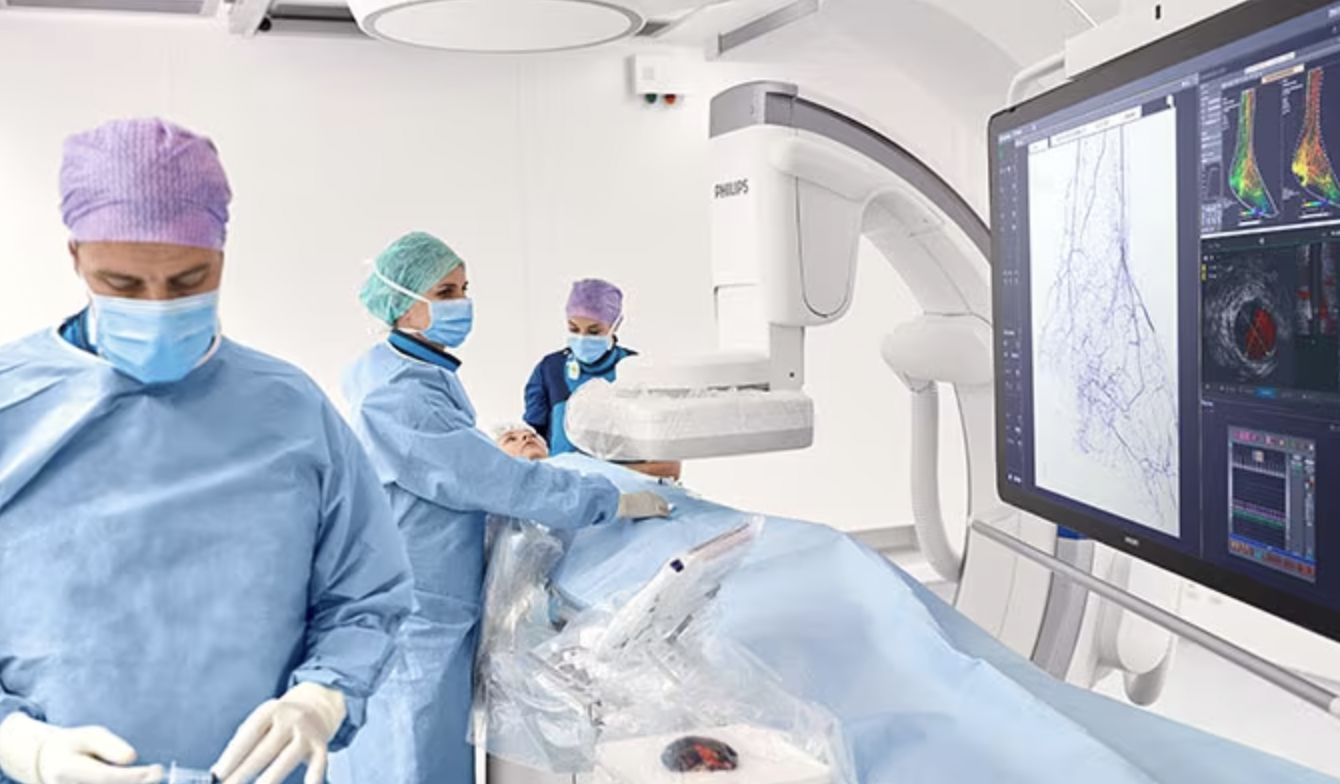
The company’s latest technology advancements in diagnostic, interventional and monitoring solutions featured at TCT 2023 include:
- Tailoring each patient’s treatment: Philips’ intravascular imaging (IVI) offering includes a range of intravascular ultrasound (IVUS) catheters, co-registration and automated measurement tools for use on Philips Image-Guided Therapy System – Azurion. Multiple clinical studies have demonstrated the patient benefits of using these tools, with a recent Journal of the American College of Cardiology (JACC) review paper referring to IVUS as “the more flexible of the options and is the one that can be utilized in almost all clinical scenarios” [1].
- Advancing the standard of care for structural heart disease: The Philips VeriSight Pro 3D Intracardiac Echocardiography (ICE) catheter produces three-dimensional images of cardiac structures, including valves, from inside their patients’ hearts. It only requires conscious sedation of the patient. The solution gives even highly experienced clinicians more confidence and control in interventional procedures. It offers 2D and 3D imaging on a single ultrasound platform – the industry-leading Philips EPIQ CVx and EPIQ CVxi, also showcased at this year’s TCT. In an observational study*, the company’s ICE catheter demonstrated comparable safety and procedural efficacy with transesophageal echocardiography (TEE), which involves passing an ultrasound transducer deep into the patient’s esophagus and requires general anesthesia.
- Advancing image-guided therapy solutions for structural heart disease: The Philips EchoNavigator 4.0 empowers cath lab teams with greater control of live fusion imaging plus new anatomical modeling and transseptal puncture guidance during minimally-invasive cardiac procedures. The solution is used with Philips Azurion and Philips’ premium cardiology ultrasound system – EPIQ CVxi.
- Increasing safety during coronary interventions: Philips’ Dynamic Coronary Roadmap software is a real-time visualization innovation that removes the need for additional contrast media during percutaneous coronary intervention (PCI) procedures. Recently published late-breaking data showed how this solution enables physicians to decrease contrast administration during procedures, making a significant contribution to both the safety and quality of PCI.
- Managing coronary artery disease with instantaneous wave-free ratio (iFR) measurements: The DEFINE-FLAIR and ReVEAL iFR trials presented at EuroPCR 2023 provided clinically relevant insights into the value of physiological assessment to optimize treatment decisions and outcomes for patients with coronary artery disease. iFR is an innovative pressure-derived index that is only offered by Philips and is considered the gold standard for physiologic measurement because it obviates the need for hyperemia, which can stress the kidneys [2,3].
- Simplifying coronary and peripheral atherectomy and lead extraction: Only the Philips Laser System – Nexcimer is compatible with catheters that have Level I clinical data for ISR (in-stent restenosis) atherectomy and that can also support interventional procedures for lead extraction, the removal of the thin insulated wires connected to cardiac pacemakers or implantable cardioverter defibrillators for the treatment of irregular heartbeats [4,5].
- Optimizing cath lab performance: Philips Interventional Applications Platform – IntraSight combines imaging, physiology and co-registration tools, enabling physicians to see clearly beyond the angiogram and ultimately complete their view of the target vessel to make fast, informed clinical decisions for optimal treatment.
Published late-breaking clinical data presented at TCT 2023
Join the presentation of Matthias Götberg, MD, PhD, interventional cardiologist and Director of the Cardiac Catheterization Lab at Skane University Hospital, Lund, Sweden, on Wednesday, October 25 at 3:12pm PT in Moscone South. Dr. Götberg presents a late-breaking clinical science session titled: Long-term Clinical Outcomes After iFR vs. FFR Guided Coronary Revascularization – Insights From the SWEDEHEART National Registry.
For more information: www.philips.com
References:
[1] Truesdell A, Alasnag M, Kaul P, et al. Intravascular Imaging During Percutaneous Coronary Intervention. J Am Coll Cardiol. 2023 Feb, 81 (6) 590–605. [2] Lawton J. et al. 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization. JACC. 2022;79(2):e21-e129. [3] Gotberg M, et al. Instantaneous wave-free ratio compared with fractional flow reserve in PCI: A cost-minimization analysis. Int J Cardiol 2021 1;344:54-59. [4] Philips Laser System Data Sheet Specifications. [5] Dippel et al. Randomized Controlled Study of Excimer Laser Atherectomy for Treatment of Femoropopliteal In-stent Restenosis: Initial ISR Results (2015). JACC 8(1): 92-101.Find more TCT23 conference coverage here
Related coverage:
TCT23 Late-breakers: Full Schedule Announced
TCT 2023 Leadership Anticipates ‘Practice Changing’ Findings Ahead of 35th Scientific Symposium
CRF Announces TCT 2023 Late-breaking Clinical Trials
MedTech Innovation Forum Leads Day One at TCT 2023
Cardiovascular Research Foundation Announces Clinical Trials Center Executive Director