Infection
An R.S.V. Shot for Infants Is in Short Supply. Here’s What to Know.
Health officials have revised their recommendations on which children should receive the new drug.
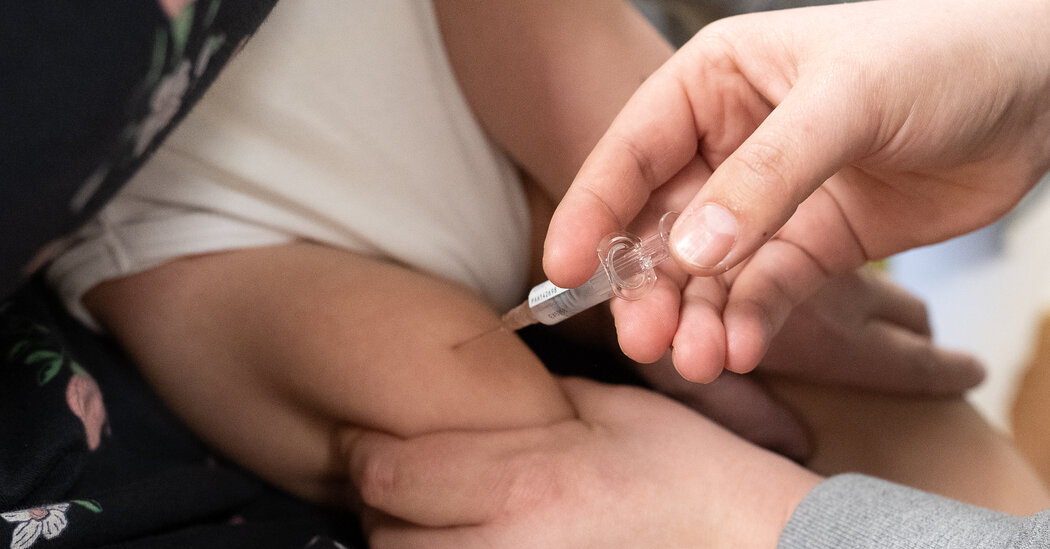
Pediatricians and parents are having difficulty accessing a new immunization used to prevent severe respiratory syncytial virus infections in infants.
The drug, called nirsevimab and sold under the brand name Beyfortus, was approved by the Food and Drug Administration in July. The Centers for Disease Control and Prevention initially recommended that it be given to all infants who are less than 8 months old, and to children 8 to 19 months old who have an increased risk for severe R.S.V.
But the demand for nirsevimab has exceeded the supply — right as R.S.V. season has started to pick up. On Monday, the C.D.C. issued an alert about the drug’s limited availability and said doctors who currently have doses should prioritize babies who are at the highest risk for severe infections: those who are less than 6 months old and infants who are immunocompromised or have an underlying health condition, such as cystic fibrosis.
To many experts, these revised recommendations make sense, given the supply restrictions. “The vast majority of the severe illness and hospitalization is in kids in the first six months of life,” said Dr. Coleen Cunningham, the chair of pediatrics at the University of California Irvine and pediatrician in chief for Children’s Hospital of Orange County. “Yes, there’s still plenty of kids getting sick beyond six months,” but they’re much less likely to become seriously ill, she said.
The shortage appears to be affecting both large hospitals and small health clinics. Whether a health care provider has nirsevimab in stock largely depends on when and how much they ordered, said Richard Hughes IV, a vaccine-law expert at the firm Epstein Becker Green.
If clinics and hospitals have not yet ordered the drug, it is unlikely that they will be able to offer it to patients this fall and winter, said Dr. Sean O’Leary, the chair of the American Academy of Pediatrics’ committee on infectious diseases.
In an email to The New York Times, a spokesman for Sanofi, which developed and manufactures nirsevimab in partnership with AstraZeneca, said that the companies are working “to accelerate additional supply and explore a number of actions to extend the manufacturing network.”
If your child qualifies for the immunization under the C.D.C.’s new guidance but your pediatrician doesn’t have it, try calling other local clinics to see if they do.
You should also confirm that your insurer will pay for it. Dr. O’Leary said that nirsevimab is included in the C.D.C.’s Vaccines For Children program, which offers free immunizations to families who otherwise might not be able to afford them. But not all private insurers are currently covering the shot, which has a list price of $495.
The supply issues are most acute for the 100 milligram dose of the drug, which is given to babies weighing more than 10 pounds. There is currently more availability of a 50 milligram dose that is approved for smaller infants and will primarily be given to those born during this R.S.V. season, which runs from October through March.
When nirsevimab was approved, it was heralded as a major advancement for infant health. In the United States, R.S.V. is the top cause of hospitalization for babies less than a year old; it results in 58,000 to 80,000 hospitalizations and 100 to 300 deaths every year among children under 5.
The drug is what is known as a monoclonal antibody therapy. It works somewhat like a vaccine, providing protection against severe disease. But the protection lasts just a few months. That’s because instead of teaching the baby’s immune system to develop antibodies against the virus, the injection delivers those antibodies directly. But once the antibodies are gone, so is the protection.
Another monoclonal antibody therapy, palivizumab, was approved in 1998 to prevent severe R.S.V. It has historically been reserved for preterm infants — who have some of the highest risk — because the drug is expensive and must be injected monthly. With the shortage of nirsevimab, the C.D.C. is now recommending that high-risk infants between 8 and 19 months be given palivizumab instead. There is an exception for American Indian and Alaska Native children who live in remote areas and have limited access to health care; they should still be given nirsevimab, if available.
An alternative option also exists for expectant mothers: This summer, the F.D.A. approved an R.S.V. vaccine for people who are 32 to 36 weeks pregnant. The antibodies they develop in response to the vaccine are passed through the placenta to the fetus, so the infant is born with protection against R.S.V.
If you have a young child who does not meet the current C.D.C. guidelines for immunization, your pediatrician is your best resource. Contact them if your child has symptoms like severe coughing, wheezing or shortness of breath.
While the shortage can be frustrating for pediatricians and parents, some experts see it as a positive sign that demand for the immunization is so high. “I think this is a reflection of success,” Mr. Hughes said. “We have this brand-new tool to protect infants, and we’ve waited so long to be able to do that.”