Infection
First rapid tests for chlamydia, gonorrhea exhibit 100% sensitivity
More than half of the estimated 374 million new sexually transmitted infections (STIs) in 2020 were either chlamydia or gonorrhea, which are often asymptomatic and co-occurring, according to the World Health Organization. Despite the prevalence, neither disease currently has a clinically available rapid test, but that could change thanks to a Penn State-led research team.
The researchers recently reported the first rapid tests for gonorrhea and chlamydia, built on a platform that could be adjusted to detect a variety of infections. Led by Dipanjan Pan, the team published their results this week in Advanced Science. Pan is the Penn State Dorothy Foehr Huck & J. Lloyd Huck Chair Professor in Nanomedicine and a professor of nuclear engineering and of materials science and engineering and of biomedical engineering.
“This work is trying to address a very important public health issue,” said Pan, who is also the director of graduate studies in the Ken and Mary Alice Department of Nuclear Engineering department, leads the Laboratory for Materials in Medicine at Penn State—which is located in the Huck Institutes of Life Sciences—and is affiliated with the Huck’s Center for Infectious Disease Dynamics.
“Infections caused by sexually transmitted diseases pose a substantial economic and health care burden worldwide. Preemptive screening and testing are key to controlling this epidemic, so the development of accurate point-of-care test for rapid and simple detection of these STIs is crucial. It will enable timely treatment, prevent further spread, raise public awareness about risks, reduce health care costs and advance health care in resource-limited areas.”
The Centers for Disease Control and Prevention recommends women under the age of 25 and older women with risk factors test for chlamydia and gonorrhea annually, and that all sexually active people should discuss testing with their health care provider. If either disease goes undetected—and untreated—it may lead to irreversible reproductive damage. Gonorrhea, which has progressively developed antibiotic resistance, can also eventually spread to blood and joints and possibly cause death.
Tests meant to deliver results at the point of care have been developed, but Pan said they often underperform and are not suitable for population screening in a physician’s office during routine family medicine visits and yearly checkups. Currently, molecular tests capable of detecting genetic material called nucleic acids from the bacteria are the standard of care, but they can be expensive and take several days to process, according to Pan.
“In spite of their excellent sensitivity and selectivity, these diagnostic approaches are expensive, time-consuming and cannot be used in a physician’s office or emergency department, making it difficult for patients and the physicians to get results immediately,” Pan said.
“The development of a point-of-care diagnostic method based on nucleic acid detection with high sensitivity, specificity and usability is urgently needed. Moreover, since co-infections occur frequently—up to 50% of the time—and have similar symptoms, simultaneous identification and detection of both pathogens is more efficient and cost-effective. To combat the current epidemic of these STIs, it is vital to develop a rapid point-of-care diagnostic assay that can detect chlamydia and gonorrhea simultaneously.”
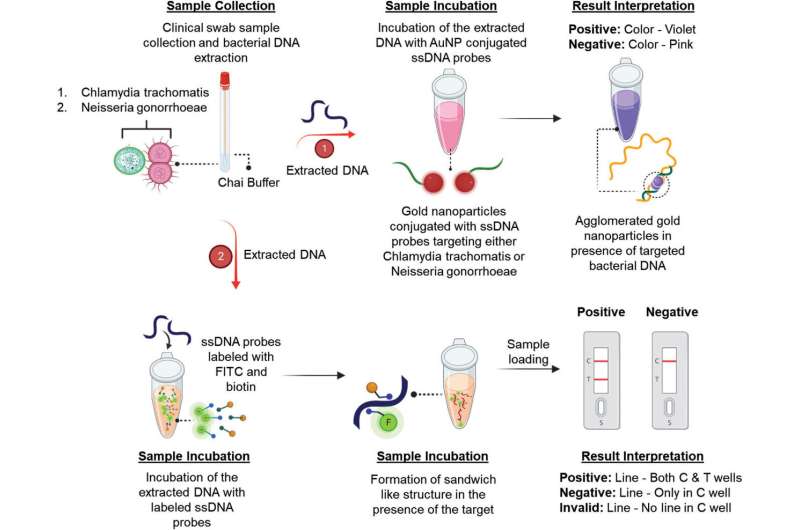
In Pan’s lab, co-authors Ketan Dighe, graduate research assistant in biomedical engineering, and Parikishit Moitra, assistant research professor of nuclear engineering, spearheaded the effort with other team members to develop a lateral flow biosensor—the same kind of paper-based biosensor used in at-home rapids tests for COVID-19—capable of detecting nucleic acids present in chlamydia or gonorrhea from cervical and vaginal swab samples in two minutes.
As with a COVID test, the sample does not require pre-processing before it is placed in a liquid that allows the contents to flow laterally from a reservoir over a sensing strip. In this case, the strip contains single-stranded oligonucleotides (ssDNAs) or fragments of nucleic acids that the researchers designed with high specificity and affinity for various genetic targets conserved among different strains of chlamydia and gonorrhea that are also less prone to antibiotic resistance, Pan said. When the ssDNA probes bind to target nucleic acid fragments in the sample flow across the strip, the strip changes colors to signify a positive test result.
The researchers also validated their work with an absorbance-based assay, or a liquid that changes color when the genetic material of interest is added to it. They accomplished this by coupling each ssDNA probe with a gold nanoparticle, which are particles with unique optical properties. When a probe binds with the target fragment, the coupled nanoparticle changes its plasmonic resonance—it changes color. The gold nanoparticle also grows in diameter and aggregates with other probe/nanoparticle sensor complexes, which can strengthen the signal and deepen the color change.
The Penn State team collaborated with Carla Rafferty, a family medicine physician with Carle Health in Illinois, and Tor Jensen, director of the Biomedical Research Laboratory for the University of Illinois Urbana-Champaign’s Cancer Center and affiliate of the Carle Foundation Hospital. When tested with 60 anonymized clinical samples collected at Rafferty’s clinic, the tests accurately detected chlamydia and gonorrhea 100% of the time, meaning they did not produce any false negatives.
The tests did have slightly lower specificity, with a rate of more than 97%, Pan said, meaning that there were about three false positive results produced. According to the U.S. Preventative Services Task Force, these rates match or exceed sensitivity and specificity for current standard-of-care molecular tests: polymerase chain reaction or nucleic acid amplification tests.
“A critical function of these ssDNAs is to identify pathogens specifically and sensitively,” Pan said. “Even if genetic concentrations are low, accurate identification is possible, minimizing the occurrence of false positives.”
According to Pan, the test also can be customized by adjusting the synthetic DNA sequences used for the ssDNAs to detect different pathogens.
“Our approach is highly universal and with small changes, the platform can be used for detection of many other infectious diseases including other sexually transmitted diseases,” Pan said. “We are currently exploring other areas in our lab.”
More information:
Ketan Dighe et al, Highly‐Specific Single‐Stranded Oligonucleotides and Functional Nanoprobes for Clinical Determination of Chlamydia Trachomatis and Neisseria Gonorrhoeae Infections, Advanced Science (2023). DOI: 10.1002/advs.202304009
Pennsylvania State University
Citation:
First rapid tests for chlamydia, gonorrhea exhibit 100% sensitivity (2023, October 27)
retrieved 27 October 2023
from https://medicalxpress.com/news/2023-10-rapid-chlamydia-gonorrhea-sensitivity.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.