Infection
The SARS-CoV-2 virus can migrate within neurons and infect the brain
The emergence of different variants of SARS-CoV-2 has produced a wide range of clinical profiles and symptoms in patients. For the first time, researchers at the Institut Pasteur and Université Paris Cité have demonstrated, in an animal model, a characteristic common to several SARS-CoV-2 variants: the ability to infect the central nervous system. The study confirms that SARS-CoV-2 is capable of infecting human neurons in vitro and migrating into axons, the nerve cell projections that carry information. The results were published on July 26, 2023 in the journal Nature Communications.
The neurological symptoms associated with SARS-CoV-2 infection have changed with the evolution of the virus and the emergence of new variants. At the start of the COVID-19 pandemic, anosmia was identified as one of the typical symptoms of infection, but it was less frequently associated with infections caused by the Omicron/BA.1 variant. Does this variability in symptoms indicate a greater or lesser affinity of SARS-CoV-2 for the nervous system?
In this study, researchers from the Institut Pasteur and Université Paris Cité have demonstrated, in an animal model, that a panel of SARS-CoV-2 variants of interest (the original strain of the virus first detected in Wuhan and the Gamma, Delta and Omicron/BA.1 variants) can enter the central nervous system and remain there during the acute phase of the infection.
The researchers observed that all these variants spread to the central nervous system and infect the olfactory bulb, a structure located in the cranial cavity that processes olfactory information before transmitting it to the cortex. “In this study, we demonstrated that infection of the olfactory bulb is common to all variants and not linked to any particular one, nor to any particular clinical manifestation such as anosmia,” explains Guilherme Dias de Melo, first author of the study and researcher in the Institut Pasteur’s Lyssavirus, Epidemiology and Neuropathology Unit. Moreover, the researchers identified a genetic sequence linked to anosmia in the ancestral (Wuhan) virus. When this genetic sequence, which encodes the ORF7ab protein, is deleted or truncated – which is the case in certain variants less likely to produce anosmia – the incidence of olfactory loss in infected animals is lower even though the degree of neuronal infection via the olfactory bulbs remains unchanged. “This suggests that anosmia and neuronal infection are two unrelated phenomena,” says Guilherme Dias de Melo. “If we follow this line of reasoning, it is quite possible that even an asymptomatic – and therefore clinically benign – infection is characterized by the spread of the virus in the nervous system.”
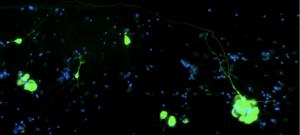
The researchers then looked at how SARS-CoV-2 reached the olfactory bulb and observed that neurons appeared to be the ideal route. An in vitro microfluidic cell culture system enabled the researchers to observe human neurons organized in a particular way. The neurons are arranged so as to allow detailed observation of the transport of molecules within the axon1.
Using this method, the researchers found that, once inside the neuron, the virus is able to move in both directions along the axon1, either in an anterograde direction, i.e. from the cell body to the axon terminals1, or in a retrograde direction, from the axons to the cell body. “The virus seems to effectively exploit the physiological mechanisms of the neuron to move in both directions. The SARS-CoV-2 variants we studied – the ancestral Wuhan variant, Gamma, Delta and Omicron/BA.1 – infect neurons in vitro and are capable of moving along axons1.”
“Through this study, we have characterized the neurotropism of SARS-CoV-2. For all the variants studied, brain infection via the olfactory bulb seems to be a common feature of SARS-CoV-2,” concludes Hervé Bourhy, last author of the study and head of the Institut Pasteur’s Lyssavirus, Epidemiology and Neuropathology Unit. “The next step will be to understand, from the animal model, whether the virus is able to persist in the brain beyond the acute phase of infection, and whether the presence of the virus can induce persistent inflammation and the symptoms described in cases of long COVID, such as anxiety, depression and brain fog.”
This research was funded by the organizations cited above and by the French Foundation for Medical Research, ANRS-Emerging Infectious Diseases and the Human Brain Project.
[1] Each neuron has an axon – a single, narrow projection that carries the electrical signal to the next cell (neuron or muscle).Source :
Neuroinvasion and anosmia are independent phenomena upon infection with SARS-CoV-2 and its variants, Nature Communications, 26th July 2023
Guilherme Dias de Melo1, Victoire Perraud1§, Flavio Alvarez2,3§, Alba Vieites-Prado4§, Seonhee Kim1, Lauriane Kergoat1, Anthony Coleon1, Bettina Salome Trüeb5, Magali Tichit6, Aurèle Piazza7, Agnès Thierry7, David Hardy6, Nicolas Wolff2, Sandie Munier8, Romain Koszul7, Etienne Simon-Lorière9, Volker Thiel10, Marc Lecuit11,12, Pierre-Marie Lledo13, Nicolas Renier4, Florence Larrous1#, Hervé Bourhy1#*
1 Institut Pasteur, Université Paris Cité, Lyssavirus Epidemiology and Neuropathology Unit, F-75015 Paris, France
2 Institut Pasteur, Université Paris Cité, Channel Receptors Unit, F-75015 Paris, France
3 Sorbonne Université, Collège Doctoral, F-75005 Paris, France
4 Institut du Cerveau et de la Moelle Épinière, Laboratoire de Plasticité Structurale, Sorbonne Université, INSERM U1127, CNRS UMR7225, 75013 Paris, France
5 Institute of Virology and Immunology (IVI), Bern, Switzerland; Department of Infectious Diseases and Pathobiology, Vetsuisse Faculty, University of Bern, Bern, Switzerland
6 Institut Pasteur, Université Paris Cité, Histopathology Platform, F-75015 Paris, France
7 Institut Pasteur, Université Paris Cité, Spatial Regulation of Genomes Laboratory, F-75015 Paris, France
8 Institut Pasteur, Université Paris Cité, Molecular Genetics of RNA viruses Unit, F-75015 Paris, France
9 Institut Pasteur, Université Paris Cité, Evolutionary Genomics of RNA Viruses Group, F-75015 Paris, France
10 Multidisciplinary Center for Infectious Diseases, University of Bern, Bern, Switzerland
11 Institut Pasteur, Université Paris Cité, Inserm U1117, Biology of Infection Unit, 75015 Paris, France
12 Necker-Enfants Malades University Hospital, Division of Infectious Diseases and Tropical Medicine, APHP, Institut Imagine, 75006, Paris, France
13 Institut Pasteur, Université Paris Cité, Perception and Memory Unit, F-75015 Paris, France ; CNRS UMR3571, 75015 Paris, France
§ These authors contributed equally
# These authors share senior authorship
* Corresponding author
dx.doi.org/10.1038/s41467-023-40228-7