Infection
Using “superhero bugs” and AI to save lives from infections
Curtin University research is aiming to combine artificial intelligence with natural, infection-fighting viruses to help save lives from an increasingly common medical emergency found in hospitals.
Common bacterial infections such as golden staph (staphylococcus) and pseudomonas can quickly put a person’s life at risk and are typically treated with medicines such as antibiotics.
However, many of the germs responsible for these infections can grow resistant to the medicines used to treat them, eventually leaving physicians with no other management options but to use higher concentrations and different combinations — which can cause serious side-effects for patients.
It’s known as antimicrobial resistance (AMR) — and Curtin School of Population Health’s Associate Professor Anthony Kicic is investigating a natural alternative to battle it.
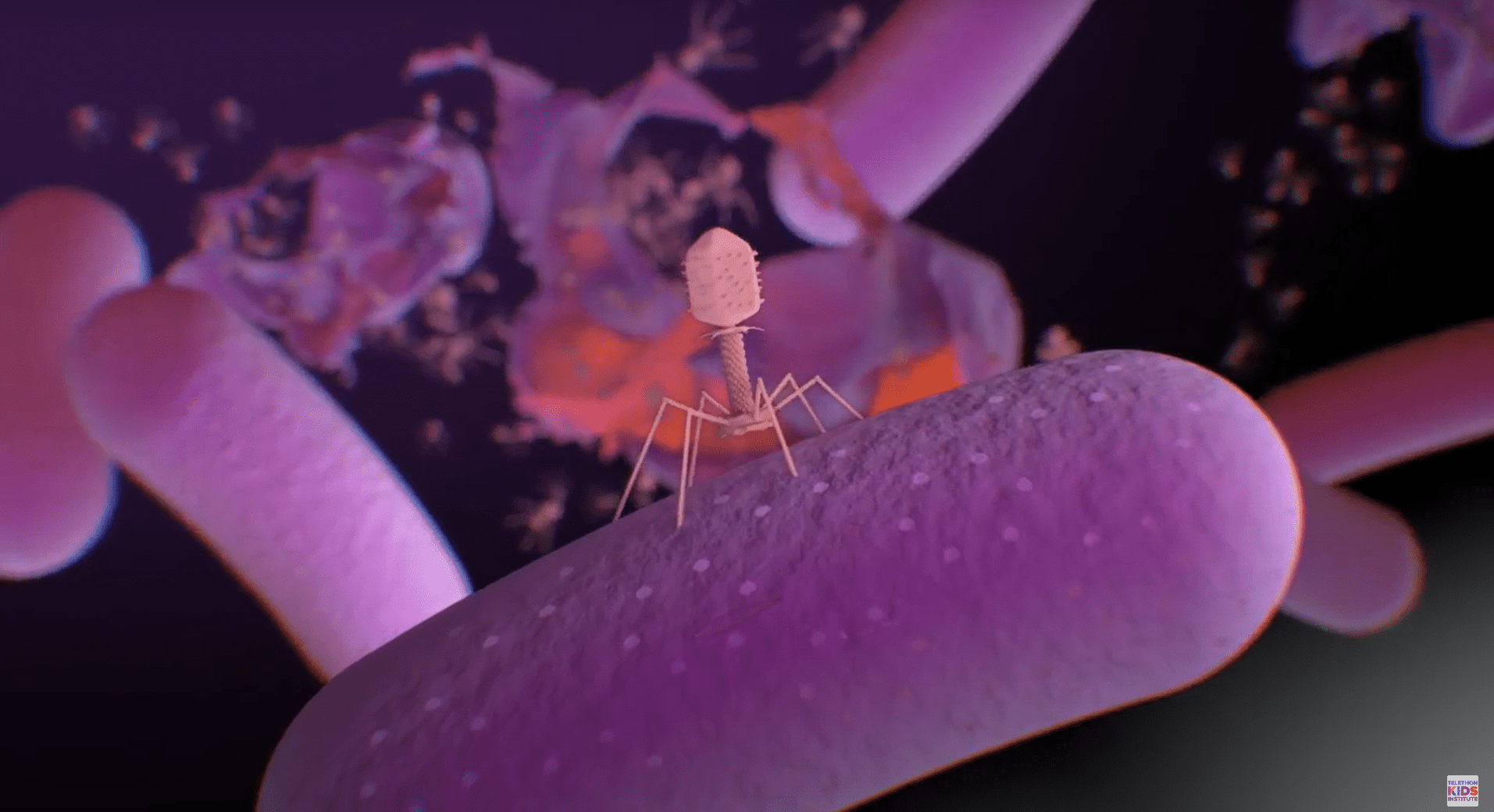
Associate Professor Kicic is researching natural viral predators of bacteria known as bacteriophages (or more commonly, phages), which can be used therapeutically.
“AMR is expected to kill more than 10 million people annually by 2050, but bacteriophage viruses are like superhero bugs found everywhere in nature,” Associate Professor Kicic said.
“They’re like something out of a movie: rather than make us sick, they seek out particular bugs, go inside them to create baby viruses, which then all burst out and kill the bacteria.
“Once all of the bacteria are killed, the phage has no host, so it too dies out.”
Phages are also a strong alternative for patients with allergies to medications, such as penicillin.
However, identifying the correct phage to combat a particular bacterial infection is a time-consuming laboratory process — and time is often in short supply when medical staff are treating a critically ill patient.
Associate Professor Kicic is partnering with the Wal-yan Respiratory Research Centre at the Telethon Kids Institute to investigate how artificial intelligence (AI) can speed up this process to save lives, with the Western Australian Government recently awarding the project an Innovation Challenge 2023 – Generative Artificial Intelligence Applications (GAIA) grant.
Associate Professor Kicic is working on an AI platform known as PHAEDRA (PHage bacteriA genomE Diagnostics Recognition via Artificial Intelligence) which will run computer simulations to quickly determine which of the thousands of phages available will be most suitable to use on a case-by-case basis.
“Australians with resistant infections are already being treated with phages, but the weakest link in the chain of service is the four to five days needed to identify the most effective phages for a specific individual’s given infection,” he said.
“Our plan is for PHAEDRA to use bacterial and phage genome sequences to identify personalised phage cocktails with the highest bacteria-killing capacity for each person’s specific infection — all within 24 hours.
“If you got a very sick patient in hospital, they may not have four days to identify a match, so if we can do it within 24 hours, we could be saving lives.”
Associate Professor Kicic is one of several Curtin researchers to achieved GAIA success, with Dr Zakir Hossain winning two grants and Dr Md Redowan Mahmoud, Dr Sonny Pham and Professor Zhonghua Sun also receiving funding for research projects.
CURTIN UNIVERSITY INNOVATION CHALLENGE 2023 – GENERATIVE AI APPLICATIONS
Associate Professor Anthony Kicic
Using in-silico generative artificial intelligence modelling to predict bacteriophage activity against specific AMR bacteria.
Dr Zakir Hossain
Generating Ultrasound images;
Generative AI to create high veracity enlarged X-ray abnormalities.
Dr Md Redowan Mahmoud
Improving the efficiency and productivity of telemental health services with a generative AI-enabled workflow automation platform.
Dr Sonny Pham
Enabling efficient and clinically accurate multimodal examinations of chest diseases.
Professor Zhongua SunClinical evaluation of a finetuned generative adversarial network (advanced artificial intelligence) model for calcium deblooming in coronary computed tomography angiography.