Cardiovascular
FDA Grants Breakthrough Device Status to Toku’s Patented Cardiovascular Risk AI (CLAiR) Platform
AUCKLAND, New Zealand–(BUSINESS WIRE)–Nov 2, 2023–
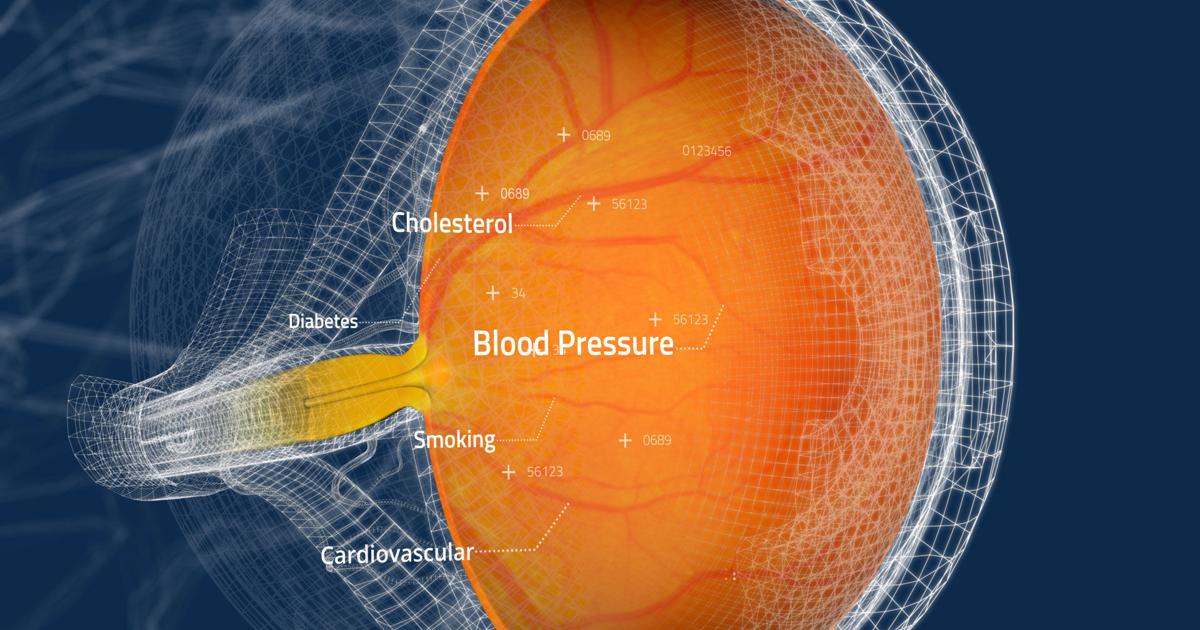
Toku, Inc., a commercial medical device company specializing in imaging technology and AI, announced that the U.S. Food and Drug Administration (FDA) has granted Breakthrough Device designation to its patented CLAiR technology. The CLAiR platform, if cleared by the FDA, will be the first medical device in the US market that can provide affordable, point-of-care and non-invasive evaluation for risk of cardiovascular disease (CVD) using fundus retinal images through a routine eye exam. Working with its partners, Toku is aiming to establish the largest network for CVD risk assessment across the US and then globally. Toku recently joined the Innovator’s Network, part of the American Heart Association’s Center for Health and Technology & Innovation. Inclusion in this group will enable Toku to collaborate easily with other cutting-edge companies creating the next generation of high-tech healthcare solutions as the Company works to bring the CLAiR platform to scale commercially, pending clearance from the FDA.