Cancer and neoplasms
Texture and color enhancement imaging improves the visibility of gastric neoplasms: clinical trial with image catalogue assessment using conventional and newly developed endoscopes
Patients and study design
This was a single-center, prospective trial. We prospectively enrolled patients who were diagnosed with gastric neoplasms (including adenoma and adenocarcinoma) through endoscopic and histological diagnosis, and who were referred to our hospital for treatment. Patients were enrolled as consecutive cases in this study to eliminate selection bias. Written informed consent was obtained from all patients.
The recruitment period was between August 2021 and July 2022. The exclusion criteria were as follows: age 8 cm) that did not fit in one endoscopic field of view, and being evaluated as inappropriate by the attending doctor for this study considering general condition. This study was approved by the Institutional Review Board of the Jikei University School of Medicine on 14 September 2020 (32–156(10,237)) and is registered with the University Hospital Medical Information Network (UMIN000042429, 16/11/2020).
Endoscopic system and setting
We used the EVIS X1 (Olympus Corporation, Tokyo, Japan) endoscopic system and high-definition endoscopes, which include a conventional endoscope (CE) (GIF-H290Z; Olympus Corporation, Tokyo, Japan) and a newly developed endoscope (NE) (GIF-XZ1200; Olympus Corporation). Regarding the image sensor, the CE uses a Charge Coupled Device (CCD), while the NE uses a high-sensitivity Complementary Metal-Oxide-Semiconductor (CMOS) which is expected to improve image quality. The EVIS X1 system can promptly change the image modalities (WLI, TXI, and NBI) via a button on the scope holder.
Texture and color enhancement imaging
TXI is a newly developed IEE that enhances the texture, brightness and color of endoscopic images obtained using WLI. First, the RGB input image is classified into a base and a detail layer. Second, the base layer is adjusted for brightness, followed by dynamic range compression (tone mapping). Subsequently, texture enhancement is applied to the detail layer to enhance subtle contrast. TXI mode 2 (TXI-2) is displayed by stacking the two layers, while the processing designed to expand the difference between red and white hues yields TXI mode 1 (TXI-1). TXI-1 is more tonally enhanced, while TXI-2 is more similar to WLI [23]; TXI is thought to enhance subtle morphological or color changes on the gastrointestinal surface caused by gastric neoplasms.
Endoscopic procedure
All endoscopic examinations were performed under sedation with intravenous midazolam (2–5 mg; Maruishi Pharmaceutical Co, Ltd., Osaka, Japan) or midazolam and pethidine hydrochloride (35 mg, pethidine; Takeda Pharmaceutical Company, Tokyo, Japan). Prior to treatment, an expert endoscopist performed an endoscopic examination, and the unmagnified endoscopic images of the lesion were stored in a middle-distant view with CE or NE using the three modalities (WLI, TXI-1 and TXI-2). On the day of treatment, images of the same lesion were stored in the same view as those in the other endoscope using the three modalities (Fig. 1). In total, we obtained six endoscopic images of each lesion using the three modalities and two endoscopes.
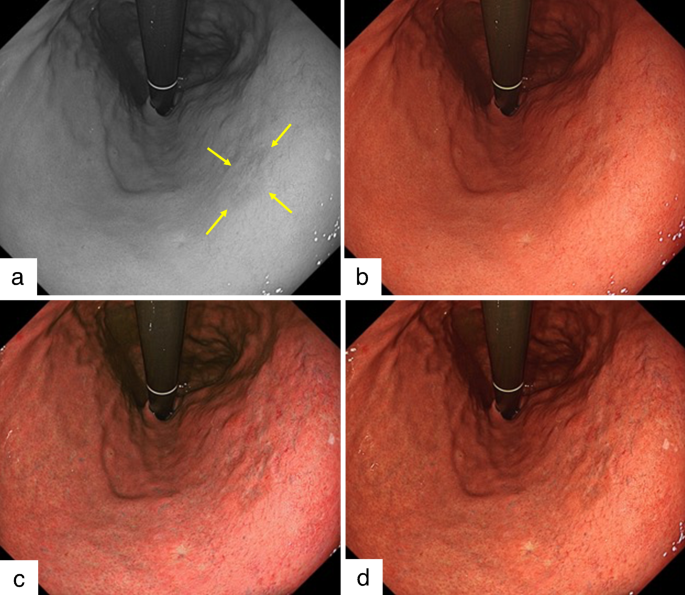
Example of early gastric cancer detected during this study. A depressed-type early gastric cancer in the lesser curvature lower body is detected using the newly developed endoscope (GIF-XZ1200, Olympus). The diagnosis of lesion margins followed the pathology finding. a. Arrows indicate lesion margins of gastric cancer in monochrome image. b. The lesion is difficult to detect in white light imaging. c. Texture and color enhancement imaging (TXI) mode 1 enhances the color and texture, and the whole image turns pinkish compared to WLI in this image. The visibility of gastric cancer is improved. d. TXI mode 2 enhances the texture, and the color tone is similar to that of WLI. The depression in the gastric cancer is enhanced
Evaluation of endoscopic images
We created an image catalogue where each gastric neoplasm had six different images. The images were randomly arranged based on a randomized table created using Excel software (Microsoft Corporation, Redmond, Washington, USA). Six endoscopists provided the visibility scales [9, 24]. All reviewers were instructed how to apply and interpret the visibility scales by an organizer (A.D.), who was not an image reviewer in this study. Visibility scale was scored based on previous reports as follows: 1, poor (not detectable without repeated careful examination); 2, fair (hardly detectable without careful examination); 3, good (detectable with careful observation); and 4, excellent (easily detectable) [10, 25]. The reviewers comprised three expert endoscopists who were certified by the board of the Japan Gastroenterological Endoscopy Society and had experience with > 100 cases of endoscopic submucosal dissection for early gastric cancer and three novices who had experience with < 100 esophagogastroduodenoscopies.
Outcomes
The primary outcome was the visibility scale score based on each modality and endoscope. The secondary outcome was the effect of lesion characteristics on the improvement of the visibility scale score for EGC. The status of H. pylori infection was defined as follows: positive (positive rapid urease test, anti-H. pylori antibody assay, or fecal H. pylori antigen assay, before eradication), eradicated (negative urease breath test or anti‐H. pylori antibody assay, post eradication), and negative (negative rapid urease test, anti‐H. pylori antibody assay, or fecal H. pylori antigen assay, without eradication) [26]. Atrophy was graded as open type, closed type, or negative according to the Kimura–Takemoto Classification [27]. The location of the neoplasm was defined as U (upper third), M (middle third) and L (lower third) according to the Japanese Classification of Gastric Carcinoma [28]. Morphology was classified according to the Paris classification [29], and histological diagnosis was based on Lauren’s classification [30]. A pathologist who did not know the result of visibility scale scores evaluated the degree of intestinal metaplasia classified as complete, incomplete, or negative according to the previous report [31].
Sample size calculation
The mean visibility scale scores for EGC were reported to be 2.54 ± 1.10 (mean ± standard deviation) and 3.28 ± 0.97 for WLI and LCI, respectively [9]. TXI was expected to improve the visibility of gastric neoplasms to the same extent as that of LCI; therefore, we calculated the sample size with an α value of 0.05 and a power of 0.90 using a two-sided test. The required number of lesions was 42. Finally, considering dropout or exclusion, we set the number of cases to 50.
Statistical analysis
All statistical analyses were performed using STATA (version 14.0; Stata Corp., College Station, Texas, United States). Quantitative parameters were compared using Student’s t test or the Mann-Whitney U test. The normal distribution was analyzed using Shapiro-Wilk test. For the secondary outcome, a two-way ANOVA was performed because the visibility scale score was evaluated repeatedly by the same reviewer. We used the visibility scale score differences between TXI-1 and WLI of NE, between TXI-2 and WLI of NE, and the following lesion characteristics for ANOVA: status of H. pylori infection, atrophy, location, size, morphology, histological diagnosis and intestinal metaplasia. We set the scale score differences as the dependent variables, and reviewers and each lesion characteristics as independent variables. The size was analyzed in two groups, including lesions ≥ 10 mm or < 10 mm. The significance level was set at p < 0.05, and Bonferroni adjustment was used when testing for repetition in ANOVA.