Blood
Mass Production of Accurate, 30-Second Blood Type Test Kit Set for Next Year
Chongqing – Gone are the days of hospital visits, lengthy waits, and cumbersome procedures for blood type identification. A simple test strip, which will soon be purchasable for home use next year, can determine one’s blood type in 30 seconds.
Southwest China’s Chongqing has introduced a revolutionary method of blood typing, an innovation that can vastly improve patient care in emergency settings, where every second counts.
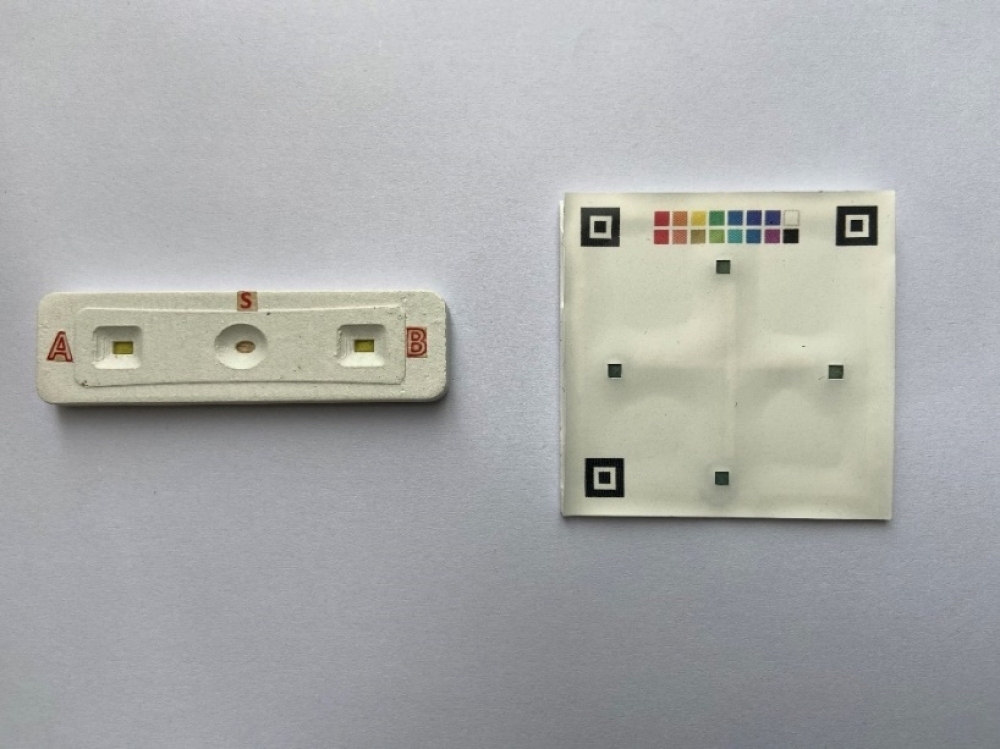
The multifunctional trauma rapid test kit. (Photo/ Luo Yang)
Luo Yang and his team developed this groundbreaking medical advancement in “Chuangzhijian – Trauma Integrated Intelligent Diagnosis Solution” 2014, a winning project at the 2023 Chongqing High-level Talent Innovation and Entrepreneurship Golden Autumn Roadshow.
A doctor and researcher, Luo was driven by the urgent need for rapid blood typing in emergency rooms. He noticed that many patients, especially those in hemorrhagic states due to accidents, were at risk due to the time-consuming process of blood type identification using traditional methods, which took over 20 minutes.
Luo combines blood type identification with infection monitoring. After two years of relentless effort, Luo and his team achieved a significant milestone: creating a portable, environment-independent rapid blood type test device.
This device could accurately determine both ABO and Rh blood types within 30 seconds and identify up to 16 blood types in under two minutes.
The team’s invention, a multifunctional trauma rapid test kit, is a pioneering development worldwide. Utilizing point-of-care testing (POCT), it offers high sensitivity, a brief testing cycle, and user-friendliness, making it invaluable in post-trauma care and life-saving situations.
Wang Zhizeng, a core team member, emphasized its global uniqueness and the significant advantages it brings to emergency medicine.
Currently, the product is fully developed and awaits further funding for clinical trials and the acquisition of a Class III medical device registration certificate.
Their efforts have attracted the attention of venture capitalists, with expectations of securing financing worth 50 million yuan (about 7 million U.S. dollars) within three years. The team envisions large-scale production by 2024.
Luo attributes his success to the support from various functional departments in Chongqing. From substantial project funding to sincere services, these departments have played a crucial role in nurturing the project. They will continue offering a suite of follow-up services to ensure the project’s growth.
Luo’s company, Chongqing Zhibai Hongjian Biotechnology Co., Ltd., is set to transform this breakthrough into a practical solution to benefit many in urgent medical situations.