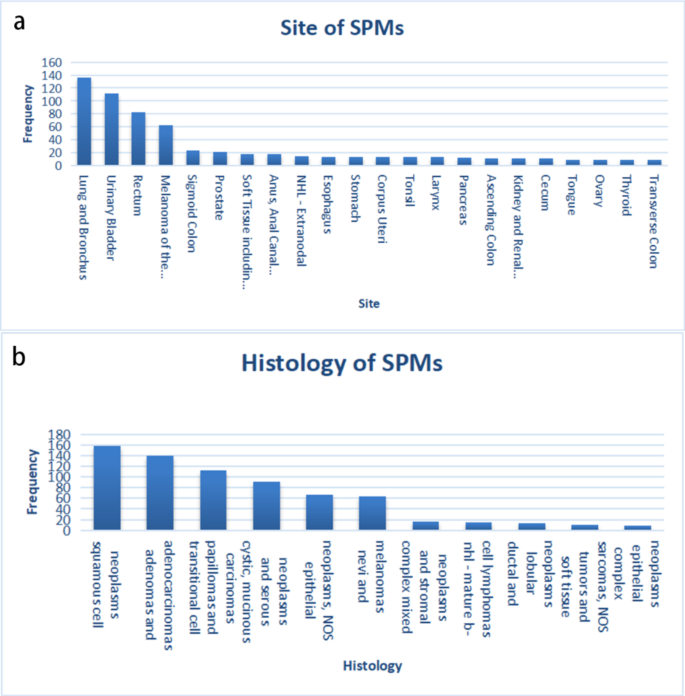
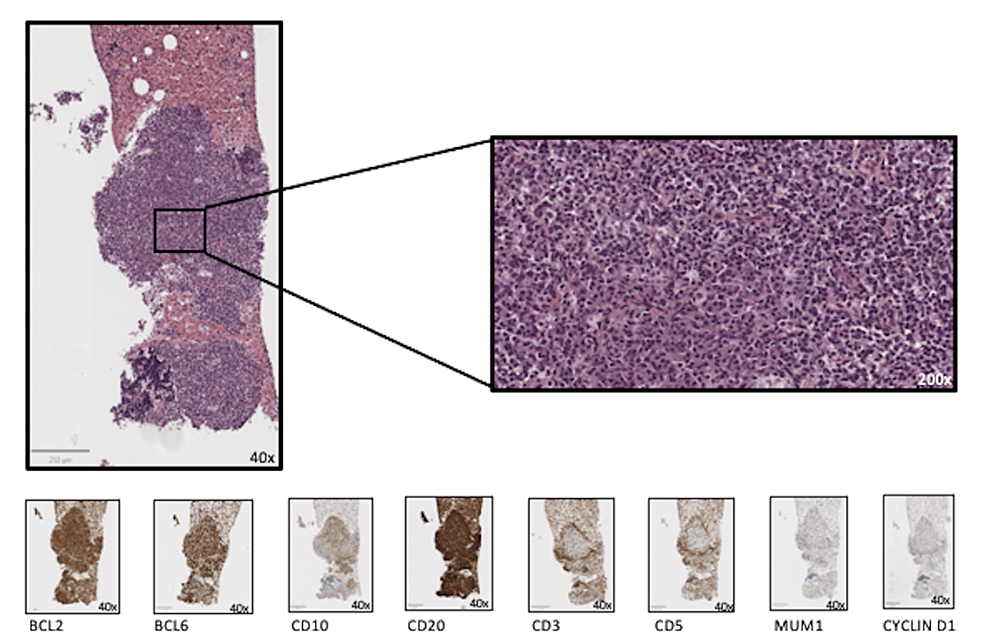
Characteristics of patients A total of 51,611 patients diagnosed with rectal cancer during 2004–2013 was obtained from the SEER database, of which 4,374 patients were diagnosed with cancer more than 6 months after the initial diagnosis of RC. To rule out caused recurrence and metastasis of RC, the patient’s data with the same histological type […]
Category Archives: Cancer and neoplasms
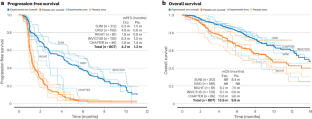
External comparator arms should be used when investigating novel therapies for gastrointestinal stromal tumor and other rare tumors to facilitate drug testing and regulatory approvals. Rare cancers, although individually uncommon, account for approximately one-quarter of all malignancies. Population-based studies have consistently shown that patients with rare cancers have worse outcomes than those diagnosed with more […]
It is important that practices provide patients with a treatment structure that really helps support an individual with any type of cancer and meets their unique needs, says Ruben A. Mesa, MD, FACP, president and executive director of Atrium Health Levine Cancer Institute (LCI) and Atrium Health Wake Forest Baptist Comprehensive Cancer Center. This transcript […]
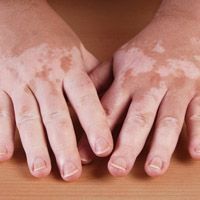
Povorcitinib treatment can lead to substantial total body and facial repigmentation for individuals with extensive nonsegmental vitiligo over all treatment groups, according to new phase 2b trial findings presented at the European Academy of Dermatology and Venereology (EADV) Congress 2023.1 The new 52-week data from EADV was announced by Incyte, and had resulted from a […]
CAMBRIDGE, Mass.–(BUSINESS WIRE)–Oct 12, 2023– Foundation Medicine Inc. today announced that it has received approval from the U.S. Food and Drug Administration (FDA) for FoundationOne ® CDx and FoundationOne®Liquid CDx to be used as companion diagnostics for Pfizer’s BRAFTOVI® (encorafenib) in combination with MEKTOVI® (binimetinib) for the treatment of adult patients with metastatic non-small cell […]
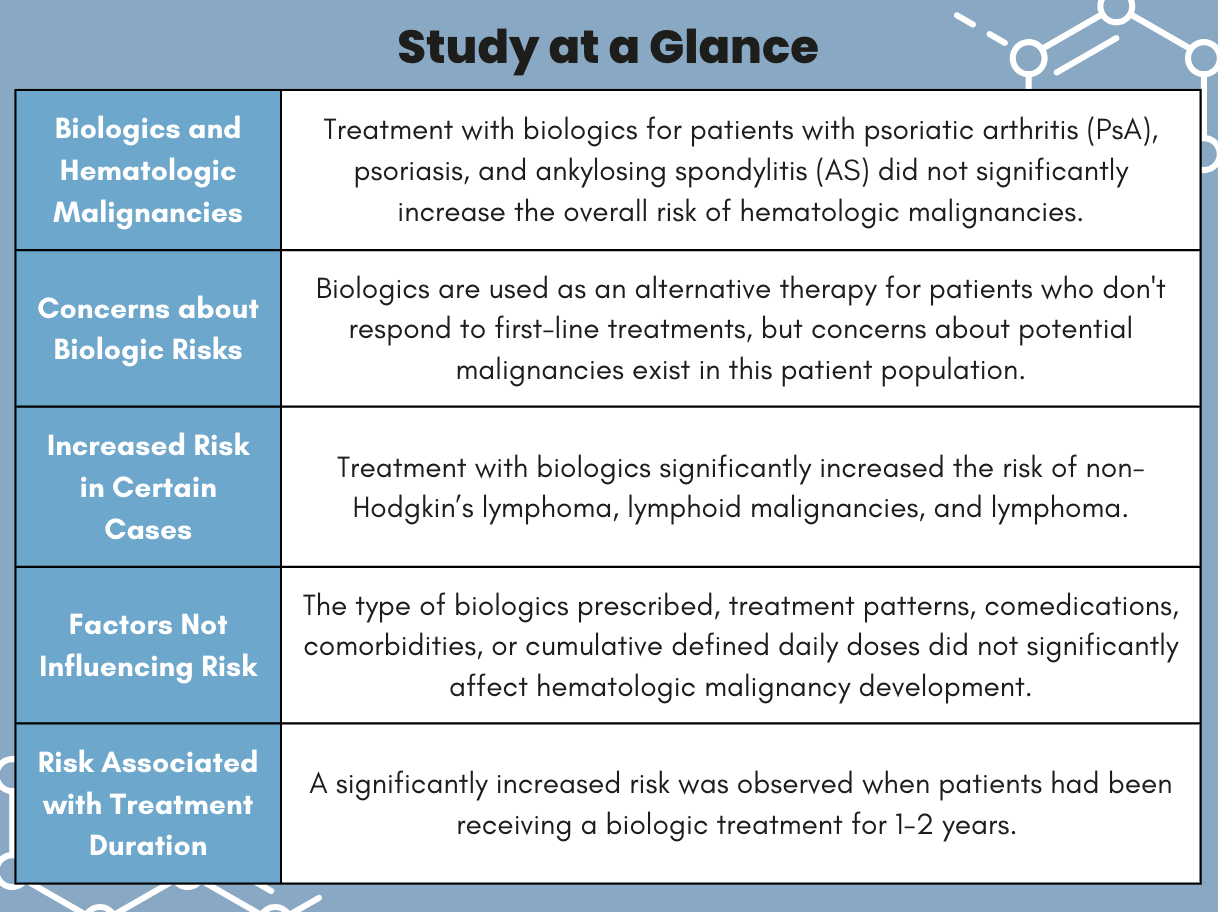
In patients with psoriatic arthritis (PsA), psoriasis, and ankylosing spondylitis (AS), treatment with biologics did not significantly increase the overall risk of hematologic malignancies. However, investigators observed a significant increased risk in those treated with biologics between 1 – 2 years and for developing certain types of lymphoma, according to research published in Biomedicines.1 Although […]
Credit: Pexels Calprotectin levels and abundance of microbial genera in primary episodes of Clostridioides difficile infection (CDI) can act as early markers of recurrent CDI, according to findings from a recent study. Investigators developed an early prediction model for recurrent CDI using clinical and analytical data including age, fecal calprotectin level, toxin B polymerase chain […]
The FDA previously approved selpercatinib in RET fusion–positive solid tumors that have progressed following prior systemic therapy in September 2022. The FDA has granted approval to the FoundationOne CDx assay as a companion diagnostic to identify patients with locally advanced or metastatic RET fusion–positive solid tumors previously treated with systemic therapies who may be eligible […]