Pegfilgrastim-cbqv received FDA approval with a single-dose, prefilled autoinjector presentation to mitigate the risk of infection resulting from febrile neutropenia among patients receiving chemotherapy for cancer in March 2023. The FDA has received a resubmitted biologics license application (BLA) supplement for an on-body injector (OBI) presentation of pegfilgrastim-cbqv (Udenyca Onbody), a pegfilgrastim (Neulasta) biosimilar, according […]
Category Archives: Cancer and neoplasms
Introduction Rhabdomyosarcoma (RMS) is the most frequent soft tissue sarcoma in children and adolescents, with around 400 new cases in the 0–19-year-old population each year in Europe,1 and 50–60 in Italy.2,3 RMS is a highly malignant tumour characterised by local invasiveness and a high propensity to metastasize.4 It can occur in any part of the […]
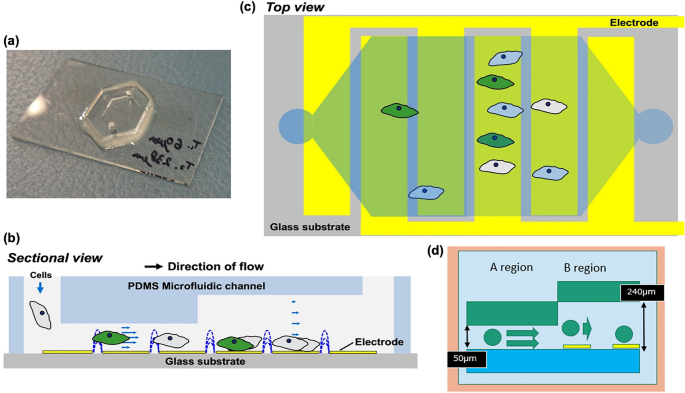
Abstract More specific screening systems for cervical cancer may become necessary as the human papillomavirus (HPV) vaccine becomes more widespread. Although p16/Ki-67 dual-staining cytology has several advantages, it requires advanced diagnostic skills. Here, we developed an automated on-chip immunostaining method using a microfluidic device. An electroactive microwell array (EMA) microfluidic device with patterned thin-film electrodes […]
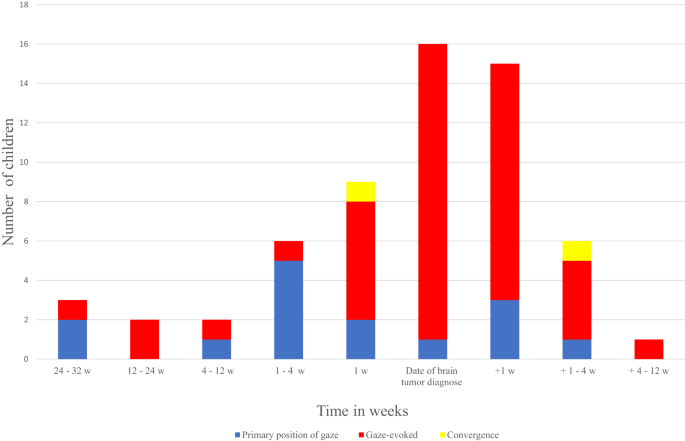
Abstract Background The aim of the study was to evaluate the prevalence, clinical characteristics, and diagnostic importance of nystagmus in children with brain tumours. Methods A nation-wide retrospective review of all children diagnosed with a brain tumour between January the 1st, 2007 and December 31st, 2017, in Denmark. Data is based on information from the […]
Bookmark 1. The GI-Genius neural network system did not improve the rate of detecting advanced colorectal neoplasia by colonoscopy in patients with a first positive fecal immunochemical test (FIT). 2. The unassisted control group had a high adenoma detection rate (ADR) at baseline. Evidence Rating Level: 1 (Excellent) Study Rundown: Colorectal cancer (CRC) screening, involving […]
Harpoon Therapeutics to Present Interim Tolerability and Response Data from Phase 1/2 Clinical Trial of T Cell Engager HPN328 at ESMO Congress 2023 News Home Monday, October 09, 2023 04:17 PM | GlobeNewswire via QuoteMedia Mentioned in this article Harpoon Therapeutics to Present Interim Tolerability and Response Data from Phase 1/2 Clinical Trial of T […]
Introduction EGFR mutations occur in 51.4% of Asian patients with lung adenocarcinomas,1 multiple co-mutations have been proven to be associated with primary resistance to EGFR tyrosine kinase inhibitors (EGFR-TKIs), among these, rare EGFR mutations that coexisted with EGFR exon 19 deletion (19DEL)/L858R are easily overlooked due to lack of function identification and clinical medication, Nevertheless, […]
Greg D Sacks Department of Surgery, New York University Grossman School of Medicine and NYU-Langone Health, New York, NY. VA New York Harbor Healthcare System, New York, NY. Hepatopancreatobiliary Service, Department of Surgery, Memorial Sloan Kettering Cancer Center, New York, NY. Paul Shin Hepatopancreatobiliary Service, Department of Surgery, Memorial Sloan Kettering Cancer Center, New York, […]
Foundation Medicine’s tissue-based comprehensive genomic profiling test is now FDA approved to identify patients with solid tumors with RET gene fusions who may benefit from treatment with Retevmo CAMBRIDGE, Mass.–(BUSINESS WIRE)– Foundation Medicine Inc. today announced that it has received approval from the U.S. Food and Drug Administration (FDA) for FoundationOne®CDx to be used as […]
New York, USA, Oct. 09, 2023 (GLOBE NEWSWIRE) — Hemophilia A Market to Exhibit Growth at a Remarkable CAGR During the Study Period (2019-2032) | DelveInsight The current therapeutic landscape of hemophilia A in the United States is driven by several approved therapies. The hemophilia A market is estimated to grow for the period 2019–2032. […]